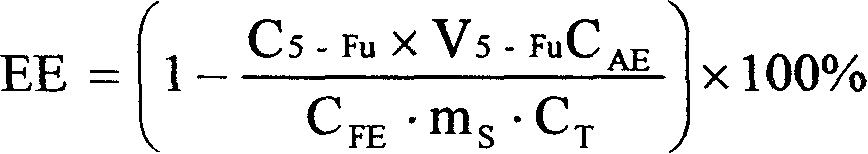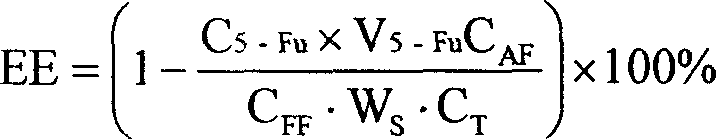Determination method for entrapment efficiency of liposome
A measurement method and liposome technology, applied in the direction of testing pharmaceutical preparations, special data processing applications, instruments, etc., can solve the problems of long time consumption, large error in results, and high requirements for instruments, and achieve short time consumption, good accuracy, and experimental results. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
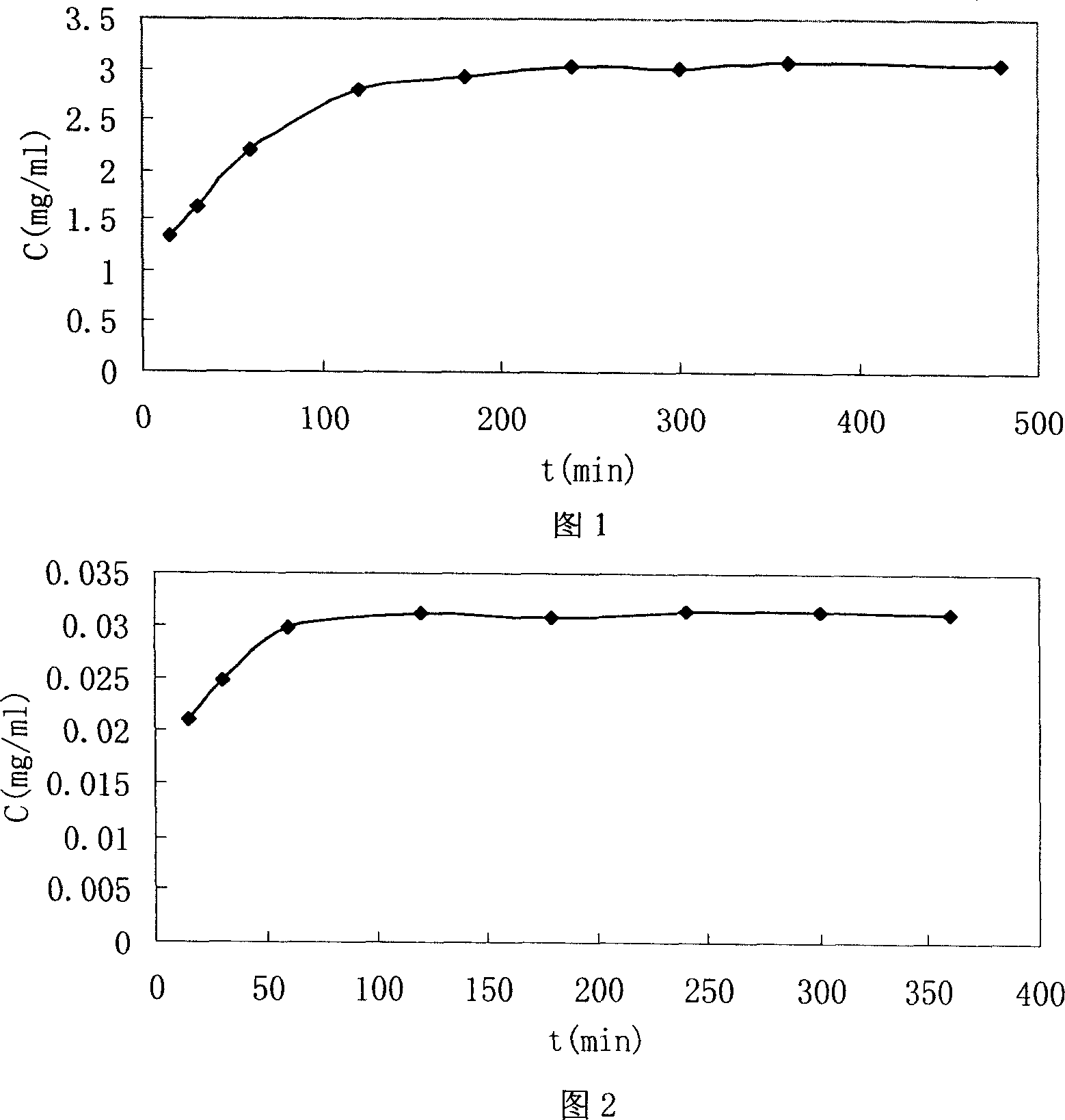
[0028] Embodiment 1 (determination of the encapsulation efficiency of the entire sennecline liposome by the reverse dialysis method of measuring the volume of the external aqueous phase with an auxiliary substance).
[0029] 1.1 Preparation of entire sennecline liposome samples
[0030] The whole-edge sennecline (A base) liposomes were prepared by dry film dispersion method. Take 0.2g of base drug A, 2g of injection-grade soybean lecithin, 0.5g of cholesterol, and 0.1g of vitamin E in a 500ml eggplant-shaped bottle, add 50ml of chloroform to dissolve, evaporate the chloroform under reduced pressure at 60°C and 50r / min to form Lipid dry film, vacuum-dried at room temperature for 12 hours to remove residual chloroform, hydrated the dry film with 50ml CBS 4.0, filled with nitrogen, shaken on the shaker to dissolve the film on the bottle wall, and then stood it in the refrigerator at 4°C for 12 hours, and finally used High-shear disperser disperses to make the whole system unifor...
Embodiment 2
[0065] Embodiment 2 (determination of the encapsulation efficiency of liposome by the centrifugation ultrafiltration method of measuring external aqueous phase volume with auxiliary substance)
[0066] 2.1 Effect of ultrafiltration on the concentration of free drug solution
[0067] Take three different concentrations (respectively 1.22, 0.50, 0.10mg / ml) of A alkali aqueous solution in three parts, 200 μL in each part, carry out ultrafiltration (Ultrafiltration unit adopts Microcon YM 100K, Millipore), and measure the filtration by HPLC respectively. The peak area corresponding to the base A in the solution and the solution before ultrafiltration. The results are shown in Table 5. It can be seen that the ultrafiltration membrane has a small amount of adsorption on the drug. When the drug concentration is large enough, it can be considered that the ultrafiltration has no effect on the concentration of the drug solution.
[0068] Table 5 The influence of ultrafiltration on the ...
Embodiment 3
[0080] Example 3 (determination of the encapsulation efficiency of the entire sennecline liposome by the reverse dialysis method of measuring the volume of the external aqueous phase with an auxiliary substance).
[0081] The test procedure and test conditions are the same as in Example 1, with phenol red replacing 5-Fu, and the test results are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com