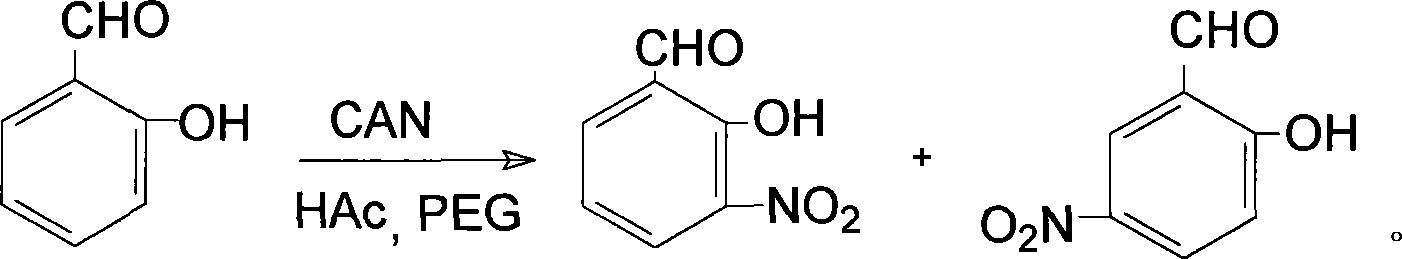
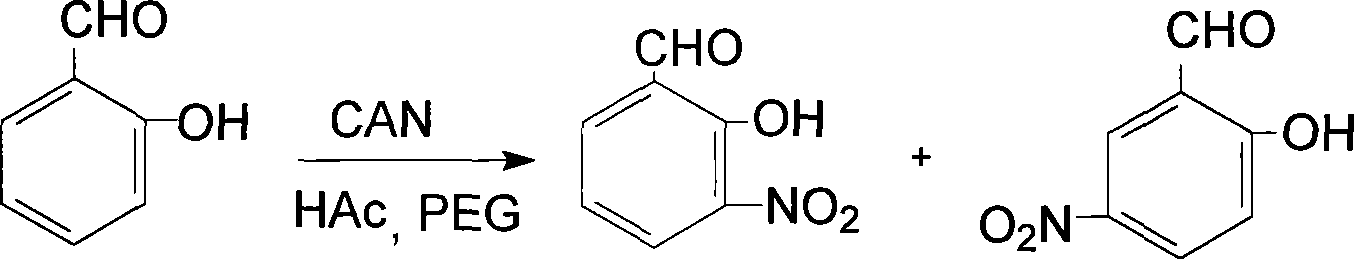
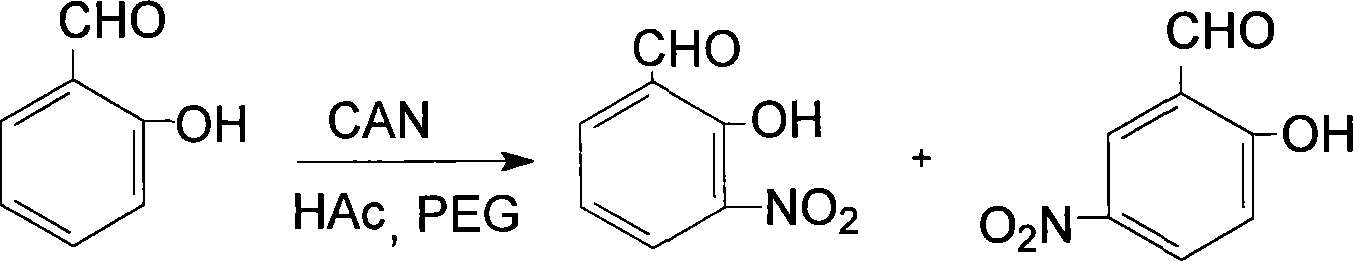
Prepn process of 3-nitro salicylaldehyde
A technology for nitrosalicylaldehyde and salicylaldehyde, which is applied in the field of 3-nitrosalicylaldehyde preparation, can solve the problems of difficult product separation, complicated operation, long reaction time and the like, and achieves convenient product separation, improved selectivity, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Add 0.61g (5mmol) salicylaldehyde, 10mL acetic acid with a volume fraction of 50%, 0.40g (1mmol) polyethylene glycol-400, 2.74g (5mmol) cerium ammonium nitrate in a 50ml Erlenmeyer flask, heat to 30°C, Reaction 1.2h, wherein, TLC traces, developer is acetone-petroleum ether, stops heating, stirs; The reaction product is poured into ice water, and the yellow solid that precipitates is 3-nitrosalicylaldehyde and 5-nitrosalicylaldehyde Mixture, add 1% sodium hydroxide solution 4.0mL (0.001mmol) in the mixture, make 3-nitrosalicylaldehyde and 5-nitrosalicylaldehyde all change into sodium salt completely, utilize two kinds of sodium salts in water solubility Difference, adjust the pH value to 3.0 with diluted hydrochloric acid, wash with water 5 times, each 30mL, can obtain 3-nitrosalicylaldehyde. Weight: 0.32g, yield: 76.6%, mp: 107-110°C, consistent with literature values, IR: Vmax (KBr tablet, cm -1 ), 3069 (-OH), 1640 (-CHO), 1627, 1472 (skeleton vibration), 1527, 1342 ...
Embodiment 2
[0016] Add 0.61g (5mmol) of salicylaldehyde, 10mL of 50% volume fraction of acetic acid, 0.60g (1.5mmol) of polyethylene glycol-400, 3.56g (6.5mmol) of ammonium cerium nitrate in a 50ml Erlenmeyer flask, and heat to 70°C , reacted for 2.0h, wherein, TLC traced, the developer was acetone-petroleum ether, stopped heating and stirring; the reaction product was poured into ice water to obtain a yellow solid, which was 3-nitrosalicylaldehyde and 5-nitrosalicylaldehyde The mixture of aldehydes, add 1% sodium hydroxide solution 4.0mL (0.001mmol) in the mixture, make 3-nitrosalicylaldehyde and 5-nitrosalicylaldehyde all change into sodium salt completely, utilize two kinds of sodium salts in water According to the difference in solubility, adjust the pH value to 4 with diluted hydrochloric acid, and then wash 5 times with 30 mL each time to obtain 3-nitrosalicylaldehyde. Weight: 0.31 g, yield: 74.3%, mp: 108.5-110°C, consistent with literature values. IR: Vmax (KBr tablet, cm -1 ), ...
Embodiment 3
[0018] In a 1500ml round bottom flask, add 61g (500mmol) salicylaldehyde, 1000mL50% (volume fraction) acetic acid, 50g (125mmol) polyethylene glycol-400, 328.8g (600mmol) cerium ammonium nitrate, heated to 50 ℃, reaction 1.6 h, wherein, TLC traces, developing agent is acetone-petroleum ether, stop heating, stir; Pour reaction product into ice water, separate out yellow solid, be the mixture of 3-nitrosalicylaldehyde and 5-nitrosalicylaldehyde , add 1% sodium hydroxide solution 400mL (0.1mmol) in the mixture, make 3-nitrosalicylaldehyde and 5-nitrosalicylaldehyde all change into sodium salt completely, utilize the difference of solubility of two kinds of sodium salts in water, Adjust the pH value to 5 with diluted hydrochloric acid, and then wash with water 6 times to obtain 3-nitrosalicylaldehyde. Weight: 31.5g, productive rate: 75.4%, mp: 108-110 ℃, consistent with literature value, IR: Vmax (KBr tablet, cm -1 ), 3069 (-OH), 1640 (-CHO), 1626, 1470 (skeleton vibration), 1526...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com