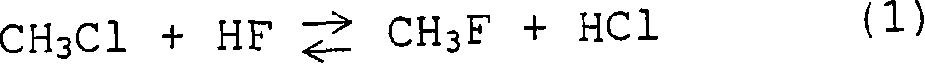Fluoromethane production process and product
A technology for fluoromethane and methane, which is applied in the field of preparing fluoromethane and can solve problems such as difficulty in separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0041] 452g of Cr(NO 3 ) 3 9H 2 O and 42g of In(NO 3 ) 3 ·nH 2 A solution of O(n about 5) in 1.2L of purified water and 0.3L of 28% ammonia water was added dropwise to a 10L container containing 0.6L of purified water within about 1 hour while stirring, while controlling the flow rates of the two aqueous solutions To make the pH of the reaction solution in the range of 7.5-8.5. The resulting hydroxide slurry was filtered, washed well with purified water, and then dried at 120°C for 12 hours. The obtained solid was pulverized, mixed with graphite, and formed into flakes using a tablet machine. These flakes were burned at 400°C for 4 hours under nitrogen flow to obtain a catalyst precursor. Catalyst precursors were loaded into an Inconel reactor and then subjected to fluorination (catalyst activation) at 350° C. under normal pressure in a flow of nitrogen-diluted hydrogen fluoride, then in a flow of 100% hydrogen fluoride to prepare the catalyst.
preparation Embodiment 2
[0044] As a catalyst support, activated alumina (NST-7, Nikki Universal Co., Ltd.) is used here, which has a central pore diameter of 50-400mm, contains at least 70% of pores with a central size distribution of ±50%, and has It has a pore volume of 0.5-1.6 ml / g, and has a purity of 99.9% by mass or more and a sodium content of not more than 100 ppm.
[0045] In 191.5g of chromium chloride (CrCl 3 ·6H 2 O) After adding 132 ml of purified water, the mixture was heated to 70-80° C. in a hot water bath to dissolve. The solution was cooled to room temperature, and then 400 g of the above activated alumina was immersed therein until the solution was absorbed into the alumina. Next, the wetted alumina was dried to harden in a hot water bath at 90°C. The hardened catalyst was dried in an air circulating hot air dryer for 3 hours. The catalyst was then loaded into an Inconel reactor and subjected to fluorination treatment (catalyst activation) at 330° C. under normal pressure in a ...
preparation Embodiment 3
[0048] Obtain the catalyst according to the method of catalyst preparation example 2, the difference is that 16.57g of zinc chloride (ZnCl 2 ) was added as the second component to the catalyst preparation example 2 of Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com