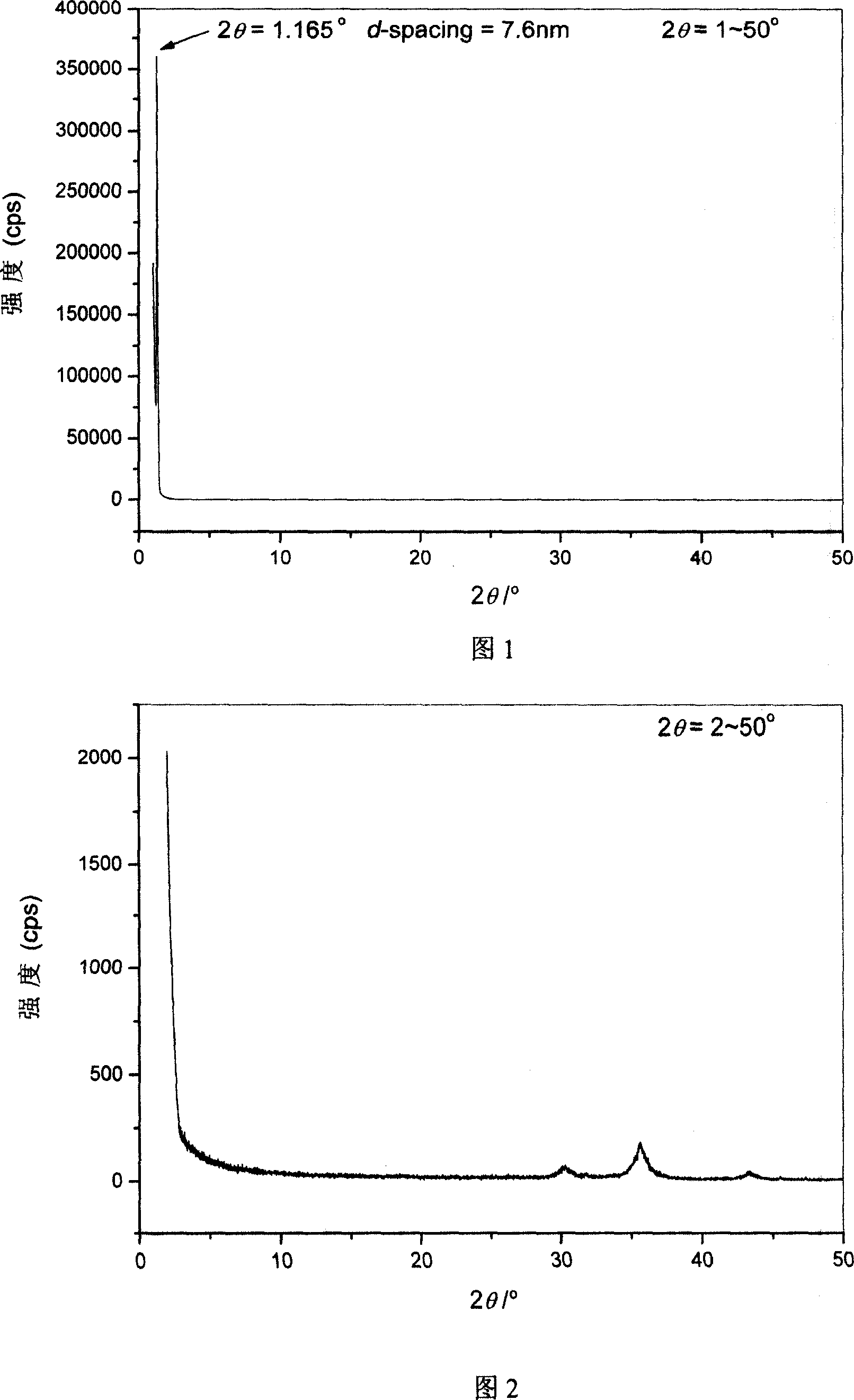
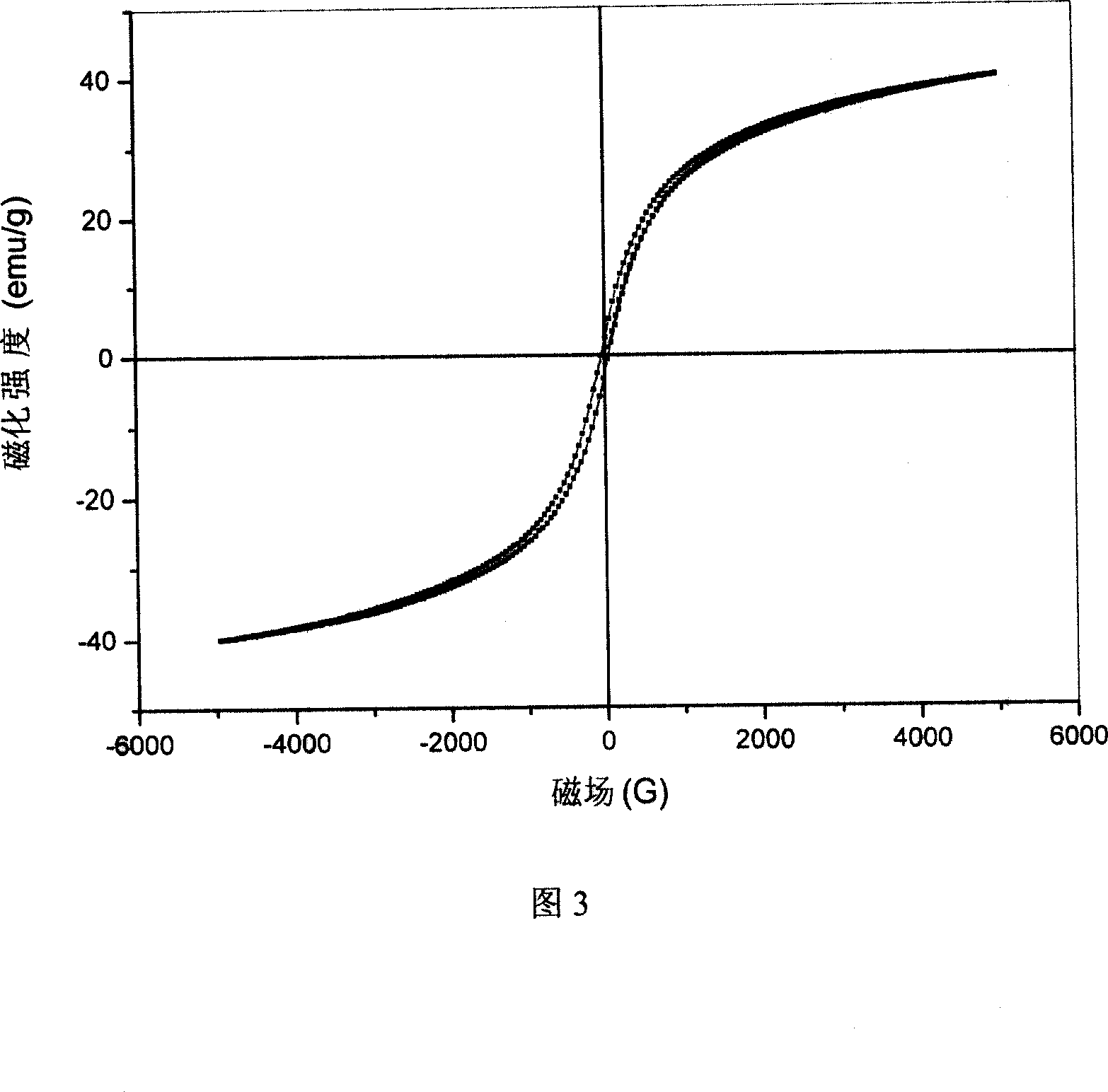
Method for preparing Fe3O4 powder with ordered nano array structure
A nano-array and orderly technology, applied in the field of Fe3O4 powder preparation, can solve the problems of harsh conditions, high price, complicated process, etc., and achieve the effect of saving energy consumption and good spatial symmetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Dissolve 0.025mol ferric trichloride and 0.015mol ferric chloride in a beaker filled with 50mL deionized water to prepare an iron salt solution with a molar concentration of 0.0008 mol iron / milliliter of water, and another 0.0018mol Sodium dodecylsulfonate was dissolved in a beaker filled with 50mL ethanol solution, and the molar concentration was prepared to be 3.6×10 -5 moles of sodium dodecylsulfonate / per milliliter of ethanol in ethanol solution of sodium dodecylsulfonate, then preheat the two to 60°C and mix them, add them to a flask with a reflux device, under electromagnetic stirring, 60 ℃ heat preservation, stand-by;
[0023] (2) 0.125mol sodium hydroxide is dissolved in 50mL water, is mixed with the sodium hydroxide solution of 0.0025 mole sodium hydroxide / every milliliter of water, then adds 50mL ethanol, is mixed with sodium hydroxide ethanol mixture, stand-by;
[0024] (3) Preheat the mixed solution prepared in step (2) to 60°C, quickly add it to the st...
Embodiment 2
[0029] (1) Dissolve 0.025mol of ferric chloride and 0.015mol of ferric chloride in a beaker filled with 50mL of deionized water to prepare an iron salt solution with a molar concentration of 0.0008 mol of iron / milliliter of water, and another 0.0009mol Sodium dodecylsulfonate was dissolved in a beaker filled with 25mL ethanol solution, and the molar concentration was prepared to be 3.6×10 -5 Moles of sodium dodecyl sulfonate / per milliliter of ethanol in ethanol solution of sodium dodecyl sulfonate, then preheat the two to 70°C and mix them, add them to a flask with a reflux device, under electromagnetic stirring, 70 ℃ heat preservation, stand-by;
[0030](2) Dissolve 0.125 mol of sodium hydroxide in 50 mL of water to prepare a sodium hydroxide solution of 0.0025 mol of sodium hydroxide / per milliliter of water, then add 25 mL of ethanol to prepare a sodium hydroxide-ethanol mixture for use;
[0031] (3) Preheat the mixed solution prepared in step (2) to 70°C, quickly add it to...
Embodiment 3
[0034] (1) Dissolve 0.025mol of ferric chloride and 0.015mol of ferric chloride in a beaker filled with 50mL of deionized water to prepare an iron salt solution with a molar concentration of 0.0008 mol of iron / milliliter of water, and another 0.0036mol Sodium dodecylsulfonate was dissolved in a beaker filled with 100mL ethanol solution, and the molar concentration was prepared to be 3.6×10 -5 moles of sodium dodecylsulfonate / per milliliter of ethanol in ethanol solution of sodium dodecylsulfonate, then the two are preheated to 80°C and mixed, then added to a flask with a reflux device, under electromagnetic stirring, 80 ℃ heat preservation, stand-by;
[0035] (2) Dissolve 0.125 mol of sodium hydroxide in 50 mL of water to prepare a sodium hydroxide solution of 0.0025 mol of sodium hydroxide / per milliliter of water, then add 100 mL of ethanol to prepare a sodium hydroxide-ethanol mixture for use;
[0036] (3) Preheat the mixed solution prepared in step (2) to 80°C, quickly add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| D value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com