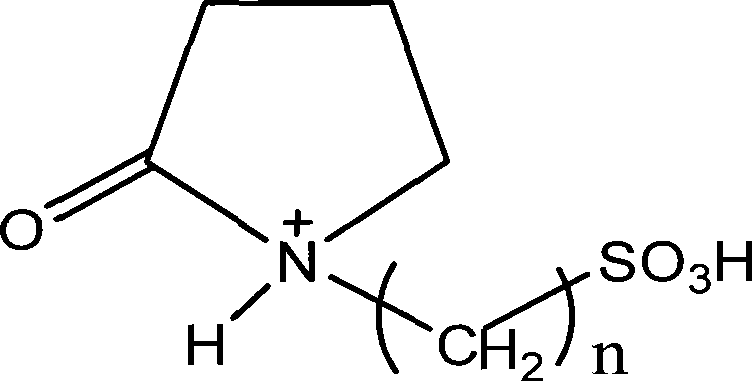
Method for catalyzing alochol acid esterization by sulfonic-acid-radical functionized ion liquid
A technology for catalyzing alkyd esters and ionic liquids, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc. Insufficient stability and other problems, to achieve the effect of low vapor pressure, reduced total production cost, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Weigh 0.1 mol of acetic acid, 0.1 mol of methanol, and 0.05 mol of ionic liquid N-(3-sulfonic acid) propylpyrrolidone bisulfate; add the ionic liquid, methanol, and acetic acid in sequence with a stirrer and a thermometer , in a round-bottomed flask with a reflux condenser, magnetically stirred, and heated to reflux at 40°C for 1 hour; stand for stratification, and take the upper esterification product, the conversion rate is 71.2%, and the selectivity is 100%; the ionic liquid is vacuum-treated Dehydrate and reuse.
Embodiment 2
[0026] Embodiment 2: Weigh 0.1mol of acetic acid, 0.1mol of ethanol, and 0.02mol of ionic liquid N-(3-sulfonic acid group) propylpyrrolidone bisulfate; add the ionic liquid, ethanol, and acetic acid in sequence with a stirrer and a thermometer , in a round-bottomed flask with a reflux condenser, magnetically stirred, and heated to reflux at 80°C for 0.5 hours; stand for stratification, and take the upper esterification product, the conversion rate is 91.1%, and the selectivity is 100%; the ionic liquid is vacuum-treated Dehydrate and reuse.
Embodiment 3
[0027] Embodiment three: take by weighing 0.1mol of acetic acid, 0.1mol of n-butanol, and 0.01mol of ionic liquid N-(3-sulfonic acid group) propylpyrrolidone bisulfate; add the ionic liquid, n-butanol, and acetic acid successively with Stirrer, thermometer, reflux condenser in a round-bottomed flask, and magnetically stirred at 100 ° C for 1 hour; stand for stratification, transfer the upper esterification product, the conversion rate is 82.5%, and the selectivity is 100%; ionic liquid It can be reused after vacuum dehydration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com