Water soluble paclitaxel product
A kind of paclitaxel, water-soluble technology, applied in the field of water-soluble paclitaxel products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
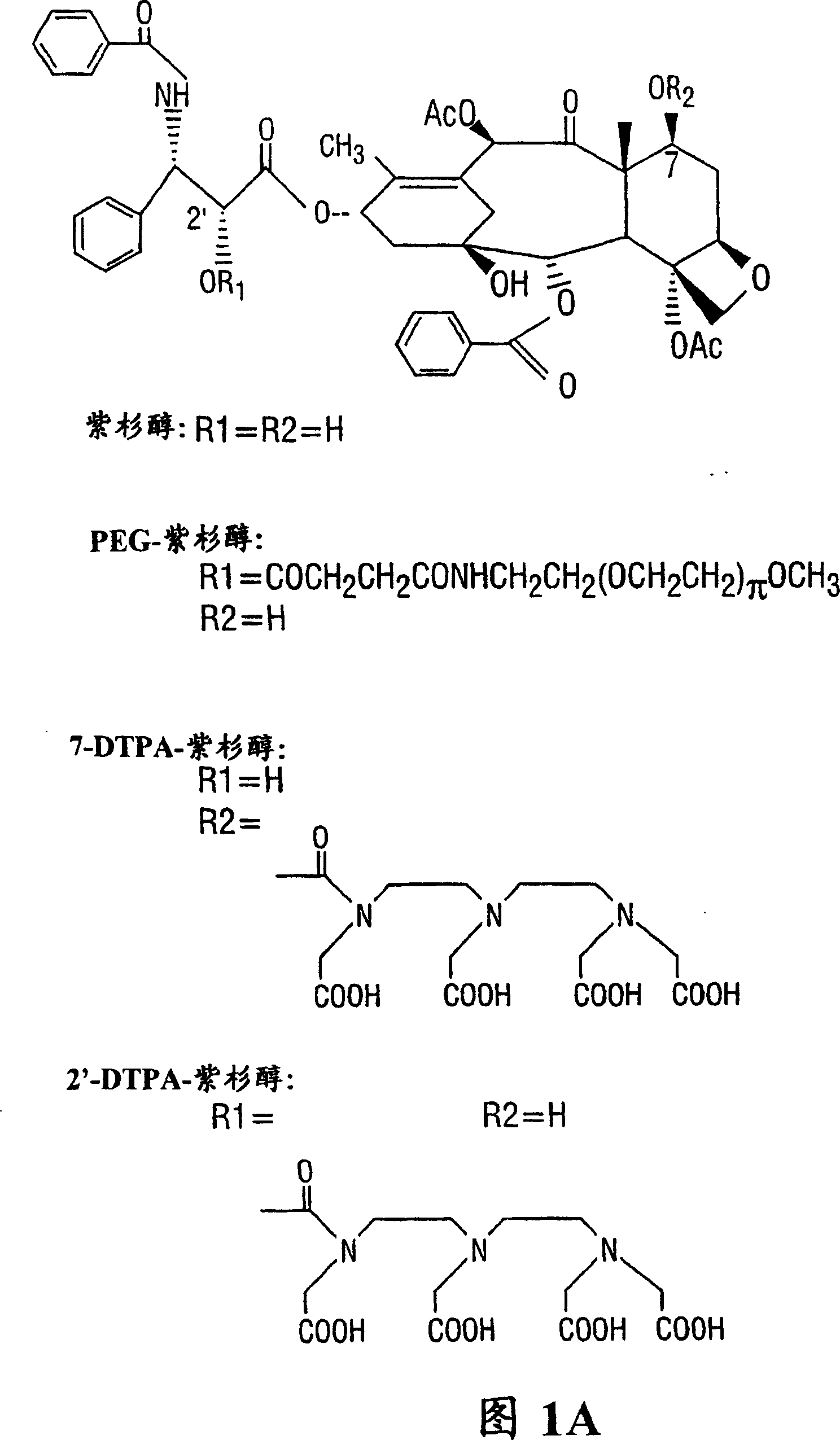
[0118] DTPA-paclitaxel
[0119] Synthesis of DTPA-paclitaxel:
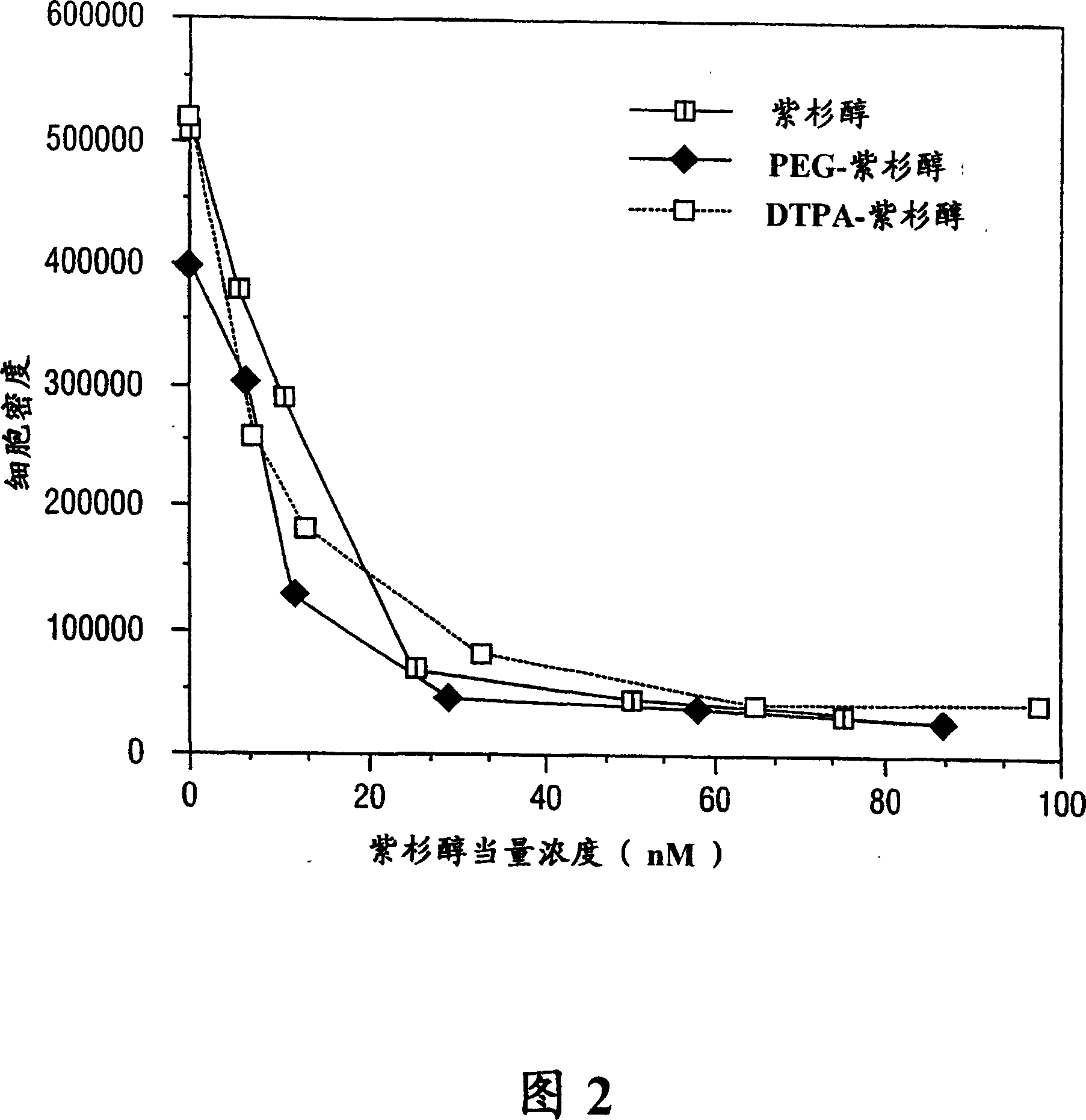
[0120] To a solution of paclitaxel (100 mg, 0.117 mmol) in anhydrous DMF (2.2 ml) was added diethylenetriaminepentaacetic anhydride (DTPA A) (210 mg, 0.585 mmol) at 0°C. The reaction mixture was stirred overnight at 4°C. The suspension was filtered (0.2um Millipore filter) to remove unreacted DTPA. The filtrate was poured into distilled water, stirred at 4°C for 20 minutes, and the precipitate was collected. C by preparative thin layer chromatography (TLC) 18 The crude product was purified on a silica gel plate and developed in acetonitrile / water (1:1). Paclitaxel R f The value is 0.34. Scrape off the R on top of paclitaxel f The band with a value of 0.65-0.75 was eluted with a mixture of acetonitrile / water (1:1), and the solvent was removed to obtain 15 mg of DTPA-paclitaxel (yield 10.4%): mp: >226°C decomposition. The ultraviolet spectrum (sodium salt solution) has the maximum absorpt...
Embodiment 2
[0137] polyglutamate-paclitaxel
[0138] This example demonstrates the conjugation of paclitaxel to a water-soluble polymer, poly(L-glutamic acid) (PG). Water-soluble polymers used as drug carriers are well known (kopecek, 1990; Maeda and Matsumura, 1989). In addition to their ability to solubilize otherwise insoluble drugs, drug-polymer conjugates can also be used as slow release sites for controlled drug release.
[0139] Synthesis of PG-paclitaxel
[0140] PG was chosen as the carrier of paclitaxel because it is easily degraded by lysosomal enzymes, stable in plasma and contains sufficient functional groups for drug adsorption. Several antineoplastic drugs have been conjugated to PG, including doxorubicin (Van Heeswijk et al., 1985; Hoes et al., 1985), cyclophosphamide (Hirano et al., 1979) and cytarabine.
[0141] PG sodium salt (MW34k, Sigma, 0.35g) was dissolved in water. The pH of the aqueous solution was adjusted to 2 with 0.2M HCl. The precip...
Embodiment 3
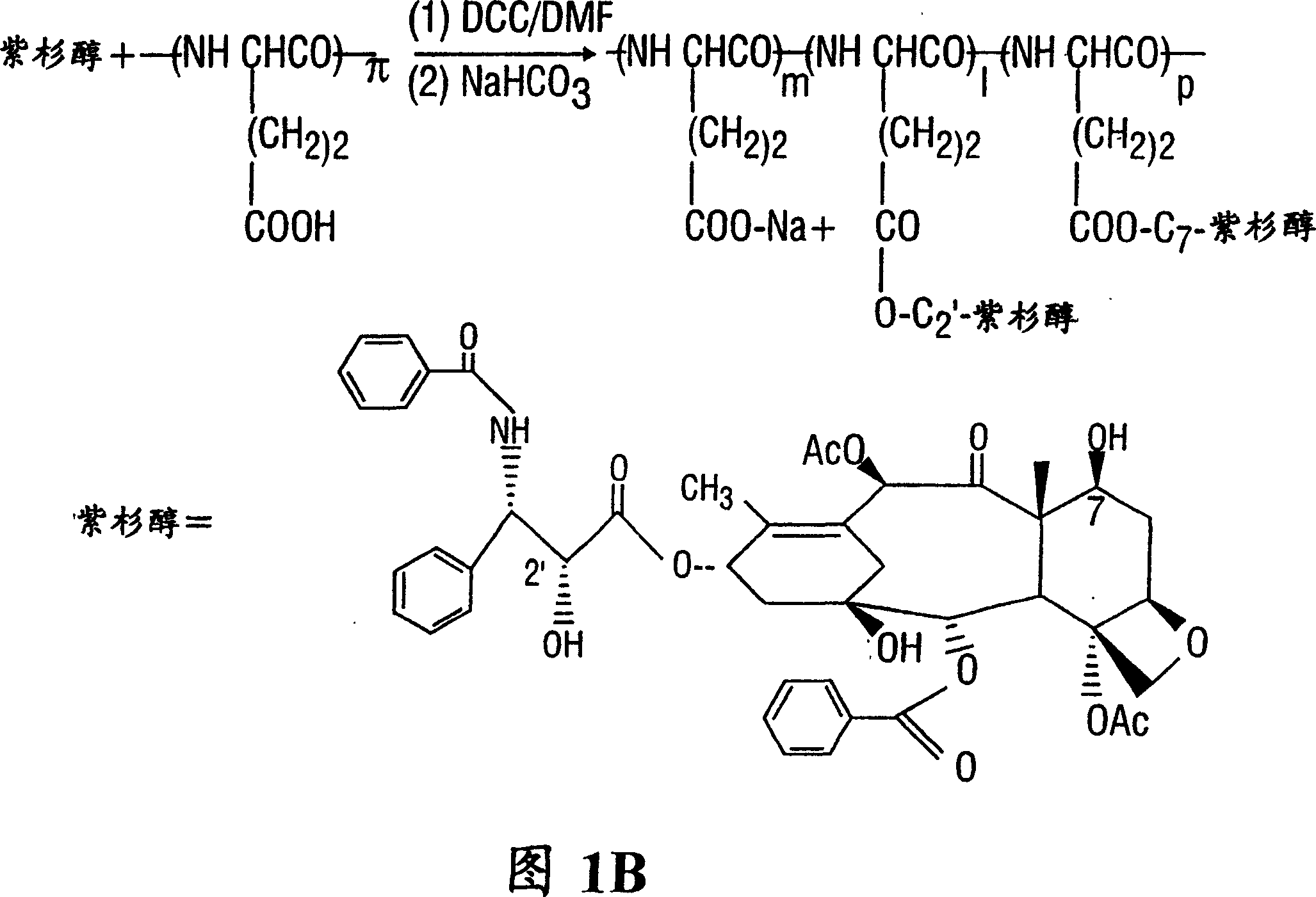
[0168] PEG-paclitaxel
[0169]Synthesis of polyethylene glycol-paclitaxel (PEG-paclitaxel)
[0170] This synthesis is done in two steps. 2'-Succinyl-paclitaxel was first prepared according to known methods (Deutsch et al., 1989). Paclitaxel (200mg, 0.23mmol) and succinic anhydride (228mg, 2.22mmol) were reacted in anhydrous pyridine (6ml) at room temperature for 3 hours. Pyridine was then evaporated and the residue was treated with water, stirred for 20 minutes and filtered. The precipitate was dissolved in acetone, water was slowly added, and the last crystals were collected to obtain 180 mg of 2'-succinyl-paclitaxel. PEG-paclitaxel was synthesized by N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ)-mediated coupling reaction. Methoxypolyoxyethyleneamine (PEG-NH 2 , MW 5000, 900 mg, 0.18 mmol) was added EEDQ (180 mg, 0.18 mmol). The reaction mixture was stirred at room temperature for 4 hours. The crude product was chromatographed on silica ge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com