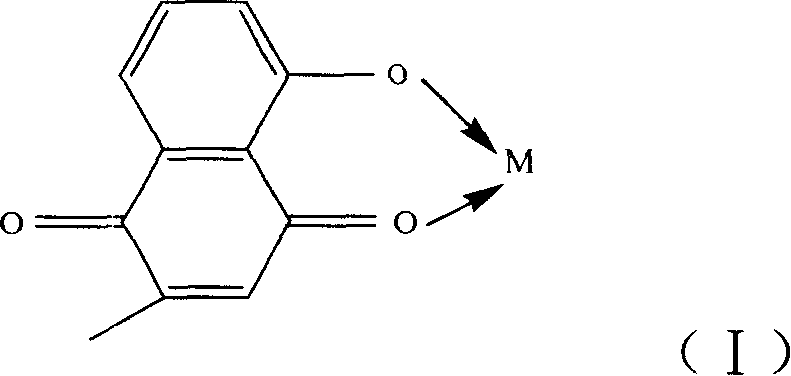
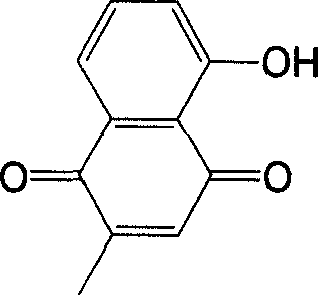
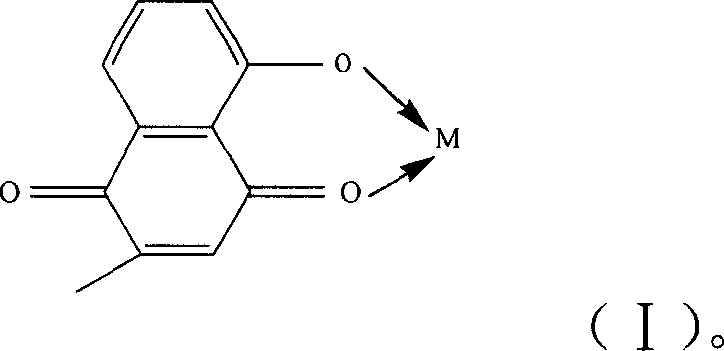
Metal complex using plumbagin as ligand, synthesizing method and usage thereof
A technology of metal complexes and plumbagin is applied in the directions of active ingredients of heavy metal compounds, compounds containing elements of Group 3/13 of the periodic table, compounds containing elements of Group 8/9/10/18 of the periodic table, etc. Solve the problems of limited skin antibacterial and anti-inflammatory effects in pharmaceutical applications, and unpublished reports of metal complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: Synthetic plumbagin Cu(II) metal complex
[0018] According to the molar ratio of 1:2, weigh 1 mol of copper acetate and 2 mol of plumbagin respectively, dissolve them in 30ml of water and 30ml of ethanol respectively, and add the copper acetate aqueous solution drop by drop under the condition of stirring at 25°C. Into the plumbagin ethanol solution, a brownish-red precipitate is formed, continue to stir for 2 hours to make it completely react, filter the solution, wash the obtained precipitate with water and absolute ethanol three times, and then place it in P 2 o 5 In the desiccator of vacuum drying to constant weight, this product is (C 11 h 7 o 3 ) 2 Cu·2H 2 O.
[0019] The synthesis method of plumbagin Co(II), Ni(II), Zn(II), Fe(II), Mn(II) and other metal complexes is the same as this method.
Embodiment 2
[0020] Embodiment 2: Synthetic plumbagin Mo (VI) metal complex
[0021] According to the molar ratio of 1:2, dissolve 1mol of molybdic acid in 25ml of water, dissolve 2mol of plumbagin in 50ml of ethanol solution, add the obtained molybdic acid aqueous solution into plumbagin ethanol solution, and heat the resulting mixed solution Reflux for 20 minutes, then drop tetraethylammonium hydroxide into it, adjust the pH value of the mixed solution to 6-7, and then reflux for 6 hours, filter out the yellow-green precipitate, and wash the obtained precipitate three times with water and absolute ethanol respectively. put again in P 2 o 5 Vacuum dried to constant weight in a desiccator, the product is MoO 2 (C 11 h 7 o 3 ) 2 .
[0022] The synthesis method of plumbagin W(VI) metal complex is the same as this method.
Embodiment 3
[0023] Embodiment 3: Synthesis of plumbagin Y(III) metal complexes
[0024] by YCl 3 ·6H 2 O: plumbagin=1:3 molar ratio, weigh 1mol of YCl 3 ·6H 2 O and 3mol of plumbagin, respectively dissolved in 30ml of water and 30ml of ethanol, will give YCl3 ·6H 2 O aqueous solution and plumbagin ethanol solution were mixed, and the resulting mixed solution was stirred for 20 minutes, then 1:1 (volume ratio) ammonia solution was added dropwise into it, and the pH value of the mixed solution was adjusted to 5-6, and then continued at 30°C. Stir for 2 hours to make it react completely, separate out a brownish-red precipitate, filter the solution, wash the precipitate with water and absolute ethanol three times respectively, and then place it in P 2 o 5 In the desiccator of vacuum drying to constant weight, this product is (C 11 h 7 o 3 ) 3 Y·2H 2 O.
[0025] The synthesis method of plumbagin La(III), Nd(III), Sm(III), Eu(III), Er(III) and other metal complexes is the same as thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com