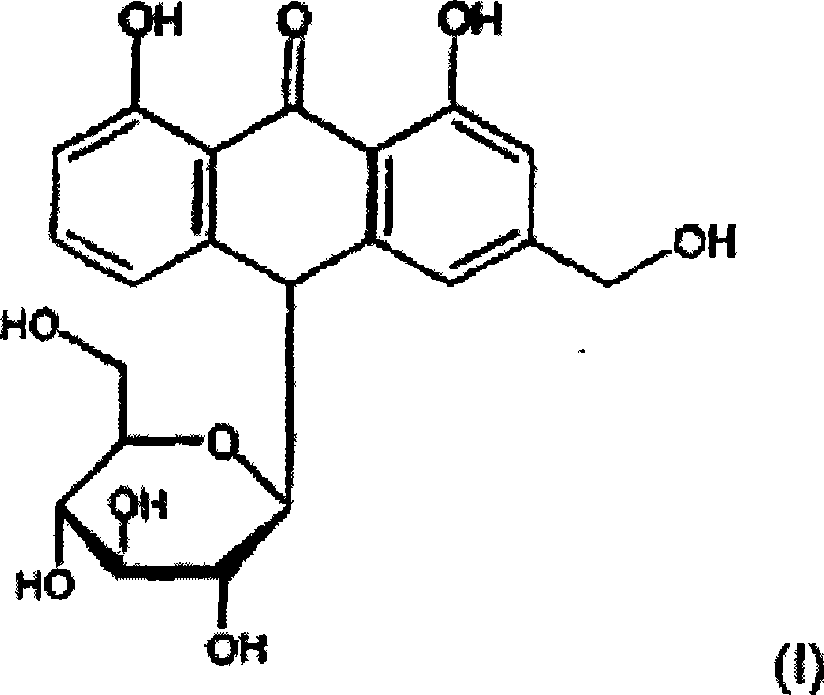
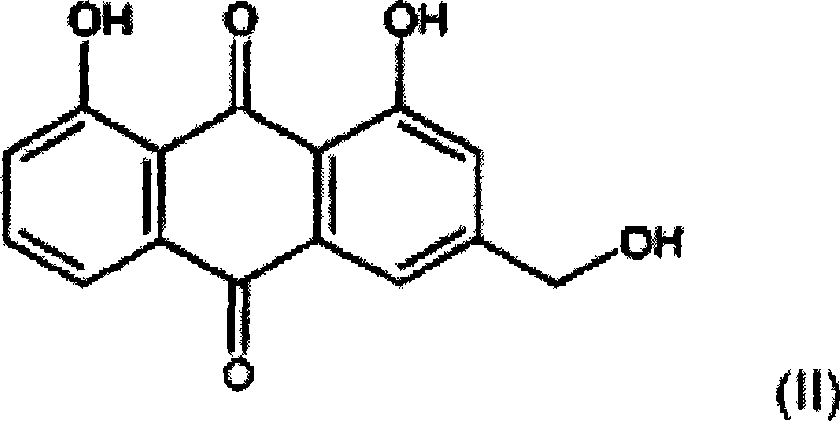
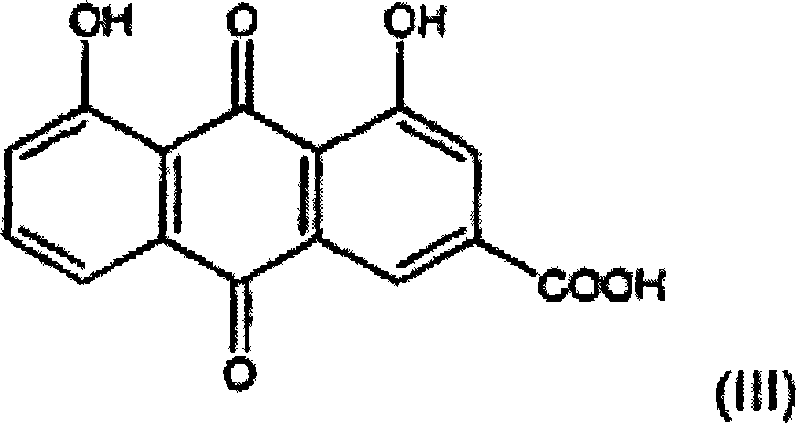
Process for preparing aloe-emodin
一种芦荟大黄素、芦荟素的技术,应用在制备大黄酸和双醋瑞因领域,能够解决复杂纯化工艺等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Crude aloin (72 g, containing 39% pure aloin) was dissolved in ethylene glycol (250 ml). This solution was poured into a one liter reactor and heated to 120°C under a nitrogen atmosphere. Upon reaching a temperature of 120 °C, HNO diluted in ethylene glycol (50 ml) was added over a period of 20 minutes 3 (7.58g). At this point, oxygen was introduced into the reactor through a sparger at a flow rate of 4 L / min. Samples were taken from the reactor every hour for analysis by HPLC to determine the completion of the reaction. After 6 hours, the reaction was complete. Under the above conditions, the conversion rate of aloin into aloe-emodin is 61%.
[0114] Then by continuously pouring the reaction mixture into water, extracting aloe-emodin with toluene or methylene chloride, evaporating toluene or methylene chloride, drying aloe-emodin (purity: 50%), precipitating aloe-emodin in ethanol, filtering aloe-emodin Aloe-emodin and dried aloe-emodin to separate aloe-emodin to ...
Embodiment 2
[0116] Crude aloin (3.24 Kg, containing 36% pure aloin) was dissolved in ethylene glycol (13.5 L) under nitrogen atmosphere. 170 g of nitric acid (dissolved in ethylene glycol) were added over a period of 5 minutes under continuous stirring. The solution was heated to 105°C. At this point, nitrogen was flushed out by introducing an oxygen flow for a period of 10 minutes. The reactor was then pressurized up to a pressure of 1.5 bar absolute by introducing oxygen. The oxygen pressure was maintained at 1.5 bar absolute for 5 min. The reactor was then depressurized to ambient pressure and cooled to room temperature. Under the above conditions, the conversion rate of aloin into aloe-emodin is 80%.
[0117] The aloe-emodin is then isolated. The reaction mixture containing suspended aloe-emodin was passed through a pressurized stainless steel filter press (8 bar absolute, 1 hour). The filter cake was then washed with half volume (7 L) of ethylene glycol under pressure (8 bar ab...
Embodiment 3
[0122] The oxidizing medium was prepared by dissolving sodium nitrite (255 g) in sulfuric acid (1.2 L). The oxidizing medium was heated to 120° C., and then aloe-emodin (100 g) was slowly added thereto. After the oxidation reaction was finished (3 hours), the reaction mixture was poured into distilled water (7.2 L) at 2°C to precipitate rhein, which was filtered and dried. Rhein with a purity of 90-95% was obtained with a yield of more than 85%.
[0123] Purification of Rhein
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com