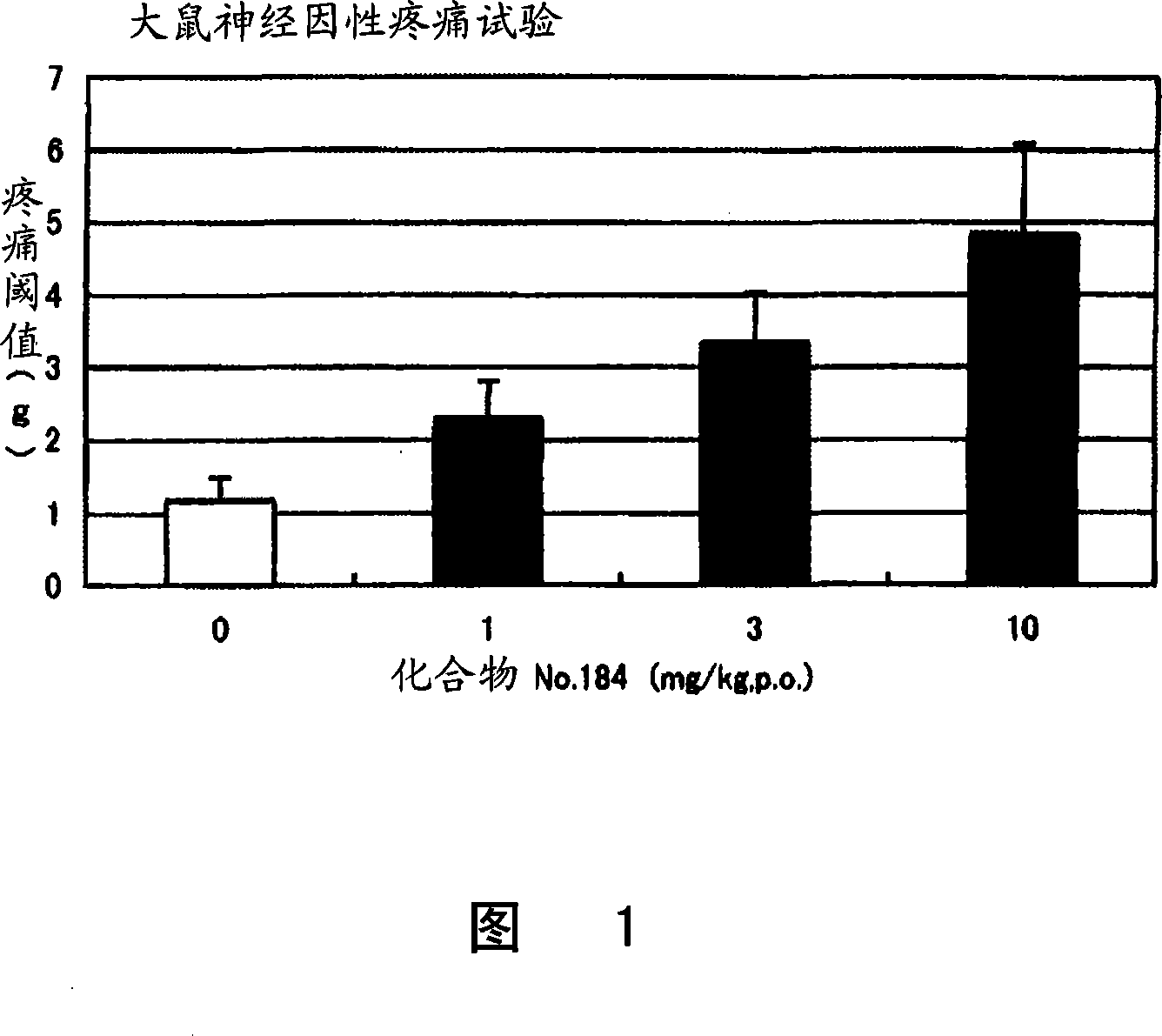
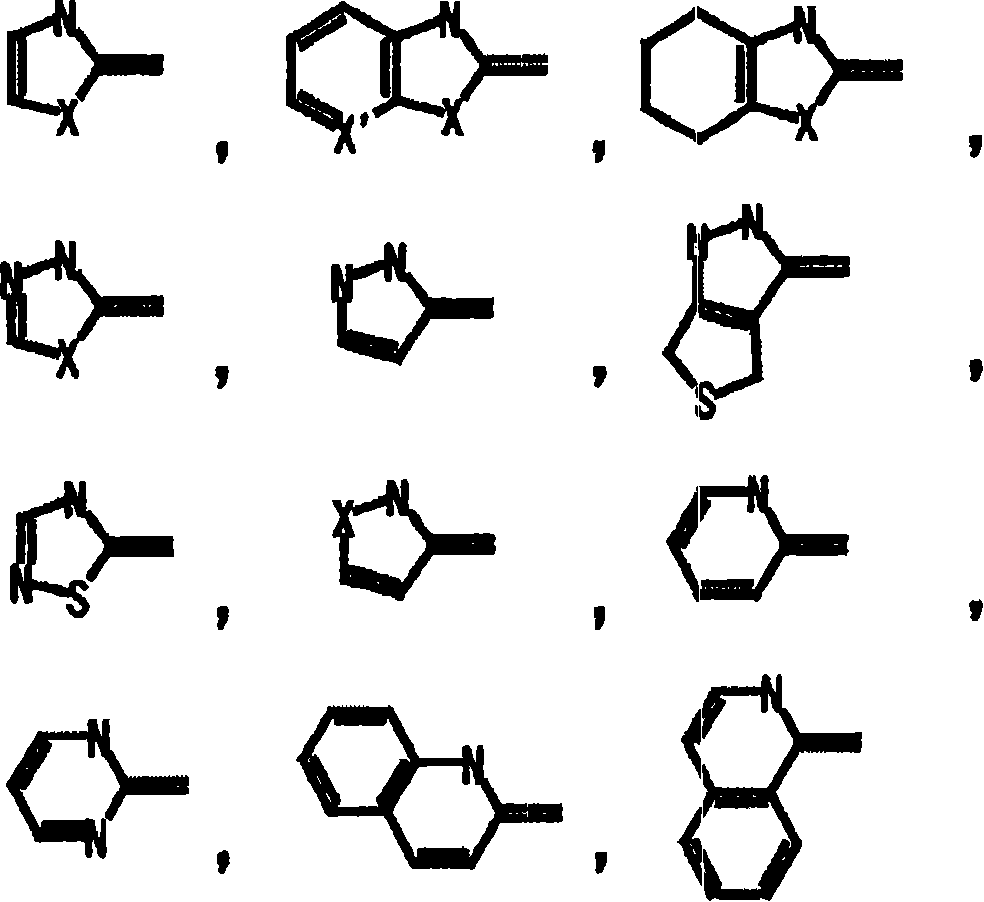
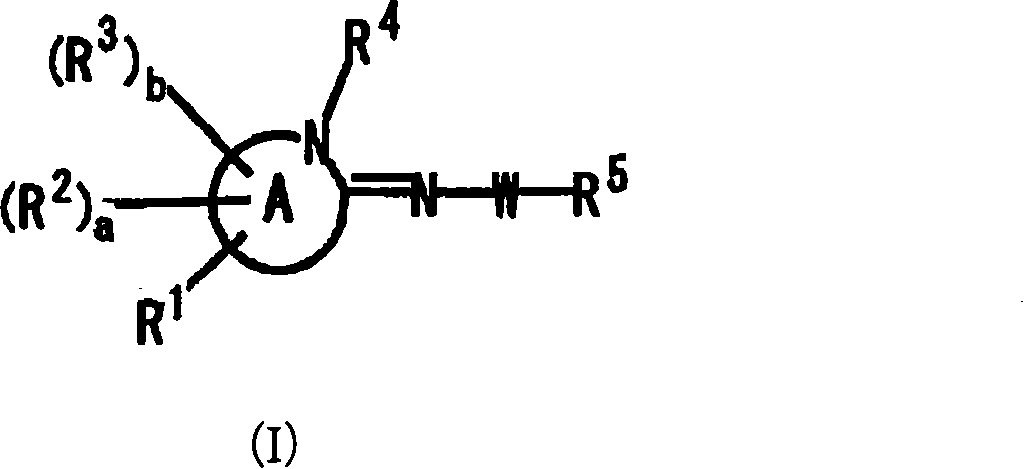Imine compound
A technology of imine compounds and alkyl groups, applied in the field of imine compounds, can solve problems such as the agonism of cannabinoid receptors that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0636] Preparation of 1-cyclopropylmethyl-2-(trifluoroacetylimino)-1,2-dihydropyridine (Compound No. 1)
[0637] 【化31】
[0638]
[0639] To a solution of 2-aminopyridine (2.0 g) and pyridine (1.9 ml) in chloroform (20 ml), trifluoroacetic anhydride (3.3 ml) was added under ice-cold conditions, and the mixture was stirred at room temperature for 3 days. The reaction solution was washed (in the order of water and saturated brine), dried (anhydrous sodium sulfate), filtered, and the filtrate was concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography (developing solvent: n-hexane-ethyl acetate = 3:1) to obtain 2-(trifluoroacetylamino)pyridine (2.3 g) as a colorless liquid.
[0640] The obtained 2-(trifluoroacetylamino)pyridine (1.3g) was dissolved in N,N-dimethylformamide (13ml), and sodium iodide (0.01g) and 60% sodium hydride ( 0.27g) and cyclopropylmethyl bromide (1.1g) were heated and stirred at 50°C for 5 hours. The reaction s...
Embodiment 2
[0643] Preparation of 1-cyclopropylmethyl-2-{3-(trifluoromethyl)benzoylimino}-1,2-dihydropyridine (Compound No. 2)
[0644] (1) 1-Cyclopropylmethyl-2-imino-1,2-dihydropyridine
[0645] 【化32】
[0646]
[0647] Dissolve 1-cyclopropylmethyl-2-(trifluoroacetylimino)-1,2-dihydropyridine (1.1g) prepared according to the preparation method shown in Example 1 in methanol (25ml), An aqueous solution obtained by dissolving anhydrous potassium carbonate (1.2 g) in water (12.5 ml) was added at room temperature, and the mixture was stirred for 2 hours. The reaction solution was extracted (ethyl acetate), dried (anhydrous sodium sulfate), and after filtration, the filtrate was concentrated under reduced pressure. The residue obtained was purified by silica gel column chromatography (developing solvent: chloroform-methanol = 18:1) to obtain 1-cyclopropylmethyl-2-imino-1,2-dihydropyridine (0.5g) as a yellow liquid ).
[0648] 1 H-NMR (200MHz, CHLOROFORM-d) d ppm; 0.31-0.37 (m, 2H), 0.58-0.67 (m...
Embodiment 3
[0656] Preparation of 1-cyclopropylmethyl-2-{3-(trifluoromethyl)phenylsulfonylimino}-1,2-dihydropyridine (Compound No. 50)
[0657] 【化34】
[0658]
[0659] According to the preparation method shown in Example 2, 3-(trifluoromethyl)phenylsulfonyl chloride was used instead of 3-(trifluoromethyl)benzoyl chloride to obtain 1-cyclopropylmethyl- as a colorless solid. 2-{3-(Trifluoromethyl)phenylsulfonylimino}-1,2-dihydropyridine.
[0660] 1 H-NMR, Mass and melting point are shown in Table 8.
[0661] Compound Nos. 3-49 and 51-59 shown in Table 1, Compound Nos. 60, 71-107, 116-174, 178-213, 215-342, 343-351, and 369-372 shown in Table 2 Compound Nos. 504-515 shown in Table 3 were prepared according to the preparation methods shown in Examples 1 and 2.
[0662] Of these compounds 1 H-NMR, Mass and melting point are shown in Table 8, Table 9 and Table 11.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Ec50 value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com