Pd radicel duplex metal selective hydrogenation catalyzer and method for preparing the same and application thereof
A hydrogenation catalyst and a selective technology, applied in the field of bimetallic butadiene selective hydrogenation catalyst and its preparation, can solve the problems of low catalyst activity and selectivity, low catalytic efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
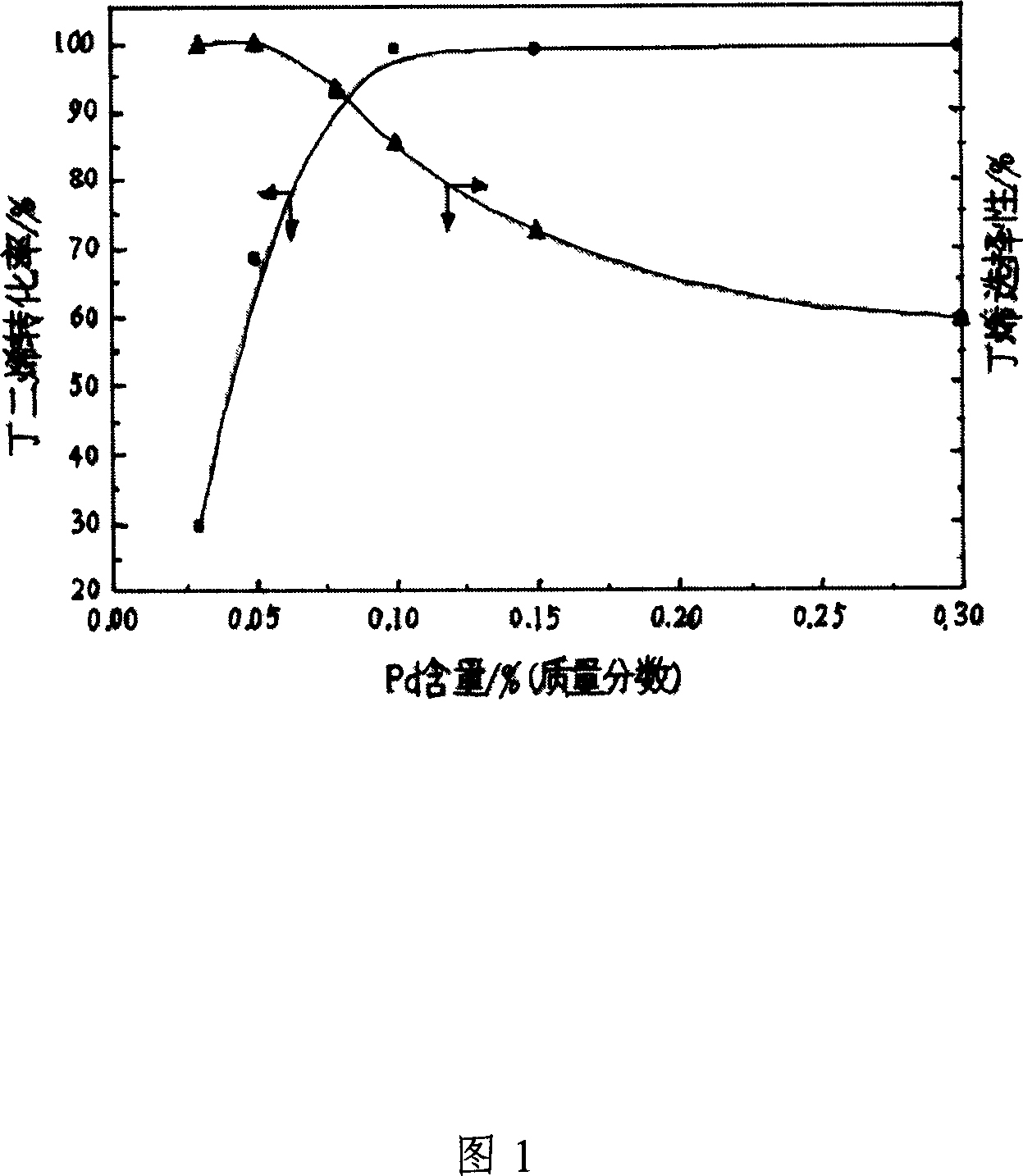
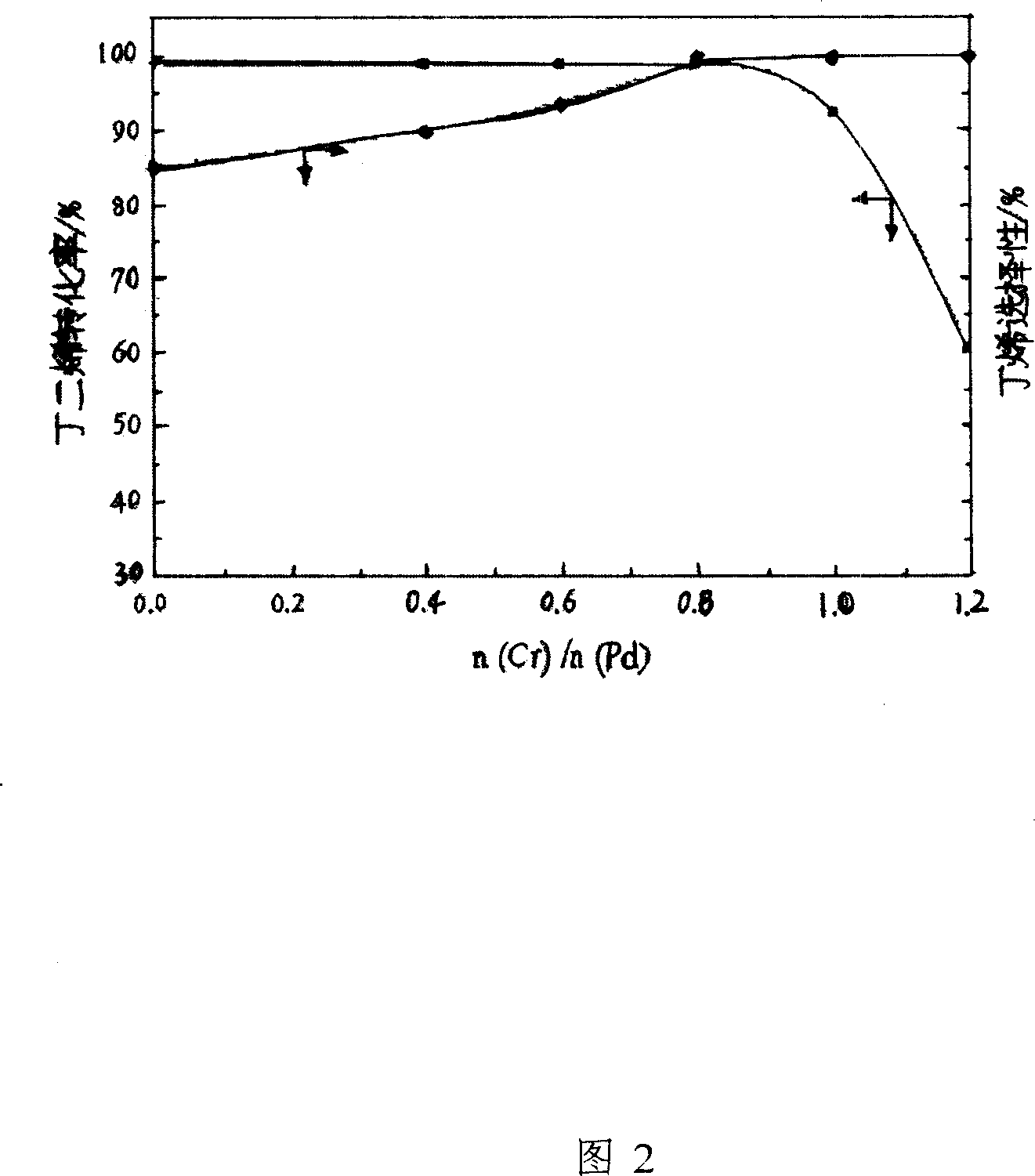
[0024] The carrier of the catalyst of the present invention is active zinc oxide, based on the total mass of the catalyst, and based on the mass content, it contains the following components: the active component is palladium metal, the content of which is 0.03%-0.3%; the catalytic component is One of chromium metal, silver metal, nickel metal, cesium metal or lead metal, and its content is 0.4%-1.2%. Figure 1 reflects the effect of palladium metal content on catalyst performance. It can be seen from Figure 1 that as the content of palladium metal increases, the active sites of the catalyst increase, and the activity of the catalyst increases. However, when the content of palladium metal increases to a certain extent, the catalyst appears to be too active, which easily leads to the deep hydrogenation of the target product butene. Decrease; In addition, the content of palladium metal is too high, which increases the cost of the catalyst and also has an adverse effect on the industr...
Embodiment 2
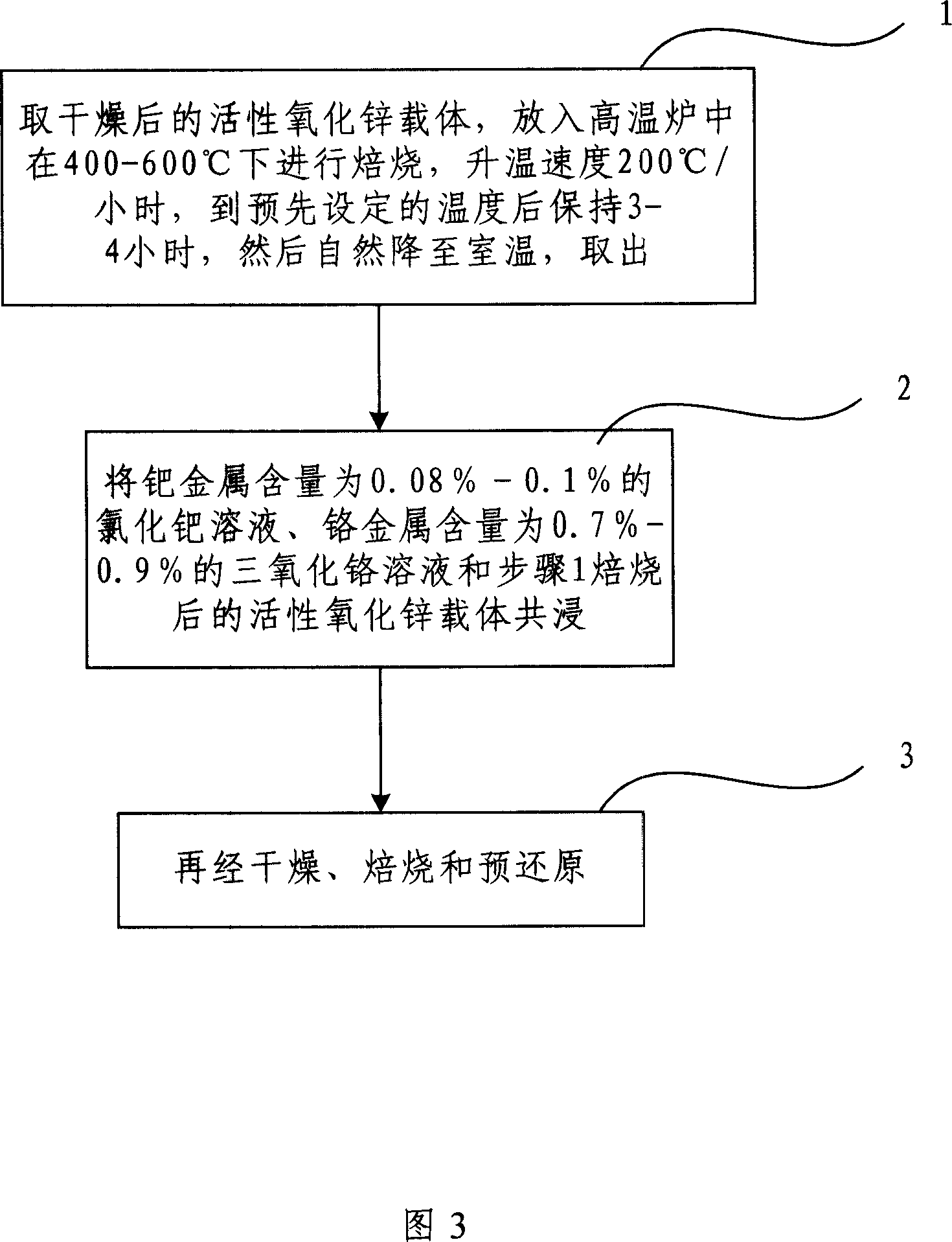
[0040]Figure 3 is a flow chart of the preparation method of the present invention. This embodiment is a preparation method of the above-mentioned catalyst, which includes the following steps:
[0041] Step 1. Take the dried active zinc oxide carrier and put it into a high-temperature furnace for roasting at 400-600°C at a heating rate of 200°C / hour. After reaching the preset temperature, keep it for 3-4 hours, and then naturally decrease to Take it out at room temperature;
[0042] Step 2. Co-impregnate a palladium chloride solution with a palladium metal content of 0.08%-0.1%, a chromium trioxide solution with a chromium metal content of 0.7%-0.9%, and the activated zinc oxide carrier roasted in step 1;
[0043] Step 3. After drying, roasting and pre-reduction.
Embodiment 3
[0045] This embodiment is a method for selective hydrogenation using the above catalyst. The method is carried out in the gas phase, the reaction temperature is 90-170°C, the reaction pressure is 0.3-0.5 MPa, and the amount of hydrogen / butadiene substance The ratio is 1.0:1 to 5.0:1, and the gas space velocity is 500-2000h -1 .
[0046] Since the reaction is carried out under gas phase conditions, as long as the pressure drop before and after the reactor does not change much, and the normal vaporization rate of the liquefied gas is ensured, the pressure change has basically no effect on the reaction, and the reaction result is stable and unchanging. Considering that the pressure of the liquefied gas tank itself is not high, and the liquefied gas is gradually vaporized, it is more appropriate to keep the pressure between 0.3-0.5 MPa during the reaction.
[0047] Figure 4 shows the effect of temperature on the hydrogenation reaction of the catalyst. It can be seen from the evaluatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com