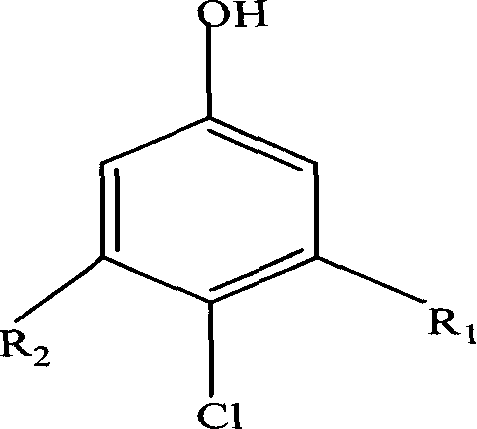
Industrial preparation method for p-chloroalkylphenols
A technology of chlorinated alkylphenols and alkylphenols, which is applied in the field of industrial preparation of chemical intermediate p-chlorinated alkylphenols, and can solve the limitations of commercialization and no significant improvement in the selectivity of para-chlorinated products. Application and other issues to achieve the effect of improving product selectivity, reducing production costs and by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of 4-chloro-3,5-dimethylphenol.
[0033] Add 2.5Kg tetrachlorethylene to the 5000ml four-necked reaction flask equipped with thermometer, stirring, condensing reflux device, and equal pressure funnel, then add 600g (5mol) 3,5-dimethylphenol, 6g iron trichloride respectively (1.0% based on the weight of 3,5-dimethylphenol), 1.22g organosulfide (0.2% based on the weight of 3,5-dimethylphenol), and 30g isopropyl ether (5% based on the weight of 3,5- weight of dimethylphenol). 675 g (5 mol) of sulfuryl chloride was added dropwise at a constant speed under stirring, and the dropping time was controlled within 3 hours. The reaction temperature was controlled at 20°C. After the dropwise addition, keep warm and continue to stir for 1 hour. Then the temperature was raised to 40° C., 250 g of tap water was added for washing, and then the layers were statically separated, and the organic solvent in the lower layer was removed for cold analysis, and sample...
Embodiment 2
[0039] Example 2: Preparation of 4-chloro-3-methylphenol.
[0040] Add respectively 2.16Kg (20mol) 3-methylphenol and 216g iron trichloride (1.0% is based on 3-methylphenol weight), 7.72g organic sulfide (0.2% based on the weight of 3-methylphenol), and 54g isopropyl ether (2.5% based on the weight of 3,5-dimethylphenol). 2.7Kg (1mol) sulfuryl chloride was added dropwise at a constant speed under stirring, and the dropping time was controlled within 3 hours. The reaction temperature was controlled at 20°C. After the dropwise addition, keep warm and continue to stir for 1 hour. Then the temperature was raised to 40°C, 1Kg of tap water was added for washing, and then the layers were statically separated, and the organic solvent in the lower layer was removed for cold analysis, and samples were taken for analysis at the same time. The analysis results are shown in Table 2:
[0041] 4-Chloro-3-methylphenol: 93.6% mol
[0042] 2-Chloro-3-methylphenol: 5.3% mol
[0043] 2,4 Ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com