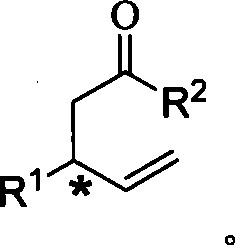
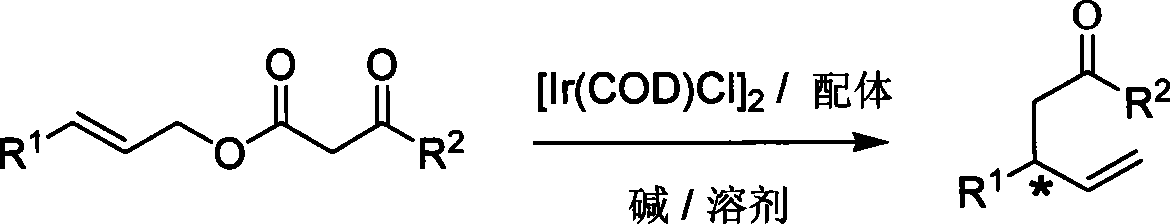
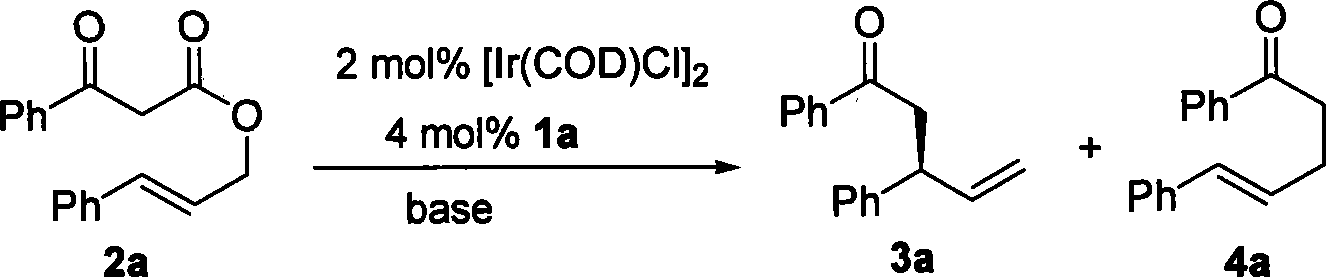Method for synthesizing 1,3-disubstitute-4-penten-1-one
A compound and disubstituted technology, applied in the field of allyl β-ketoester decarboxylation allyl alkylation reaction, can solve the problems of unstable enol silyl ether and limited application, and achieve mild reaction conditions and easy operation , the effect of high regional selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Under the catalysis of iridium complex, the research on the temperature and solvent of the allyl alkylation reaction of decarboxylation:
[0023]
[0024] Wherein, mol means mole, and base means base.
[0025] serial number
[0026] 8
[0027] Among them, THF is tetrahydrofuran, Et 2O is diethyl ether, DME is dimethylethylene ether, Toluene is toluene, DCM is dichloromethane, DBU is 1,8-diazabicyclo[5,4,0]undec-7-ene, DBN is 1,5-diazabicyclo[4,3,0]non-5-ene, and BSA is N,O-bis(trimethylsilyl)acetamide.
Embodiment 2
[0028] Example 2: Research on the allyl alkylation reaction of decarboxylation of different ligands under the catalysis of iridium complexes:
[0029]
[0030] 1a R 3 , R 4 =Ph 1dR 3 , R 4 =Ph
[0031] 1bR 3 , R 4 = 2-Naphthyl
[0032] 1c 3 , R 4 =2-MeO-Ph
[0033] Where Ph is phenyl, Naphthyl is naphthyl and MeO is methoxy.
[0034] serial number
Ligand
time (h)
Yield (%)[b]
3a / 4a
ee(%)
1
1a
16
83
99∶1
95
2
1b
36
21
99∶1
93
3
1c
24
65
>99∶1
96
4
1d
36
45
94∶6
70
[0035] Example 2: Allyl β-ketoester decarboxylated allyl alkylation reaction under the catalysis of iridium complex
[0036]
[0037] Add [Ir(COD)Cl] sequentially to a dry reaction tube 2 (0.004mmol), chiral ligand (0.008mmol), n-propylamine (0.5mL) and THF (0.5mL),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com