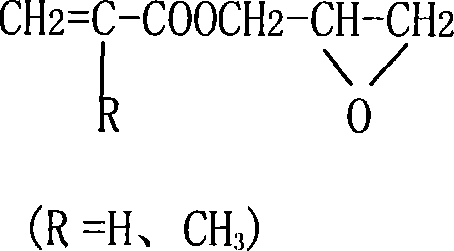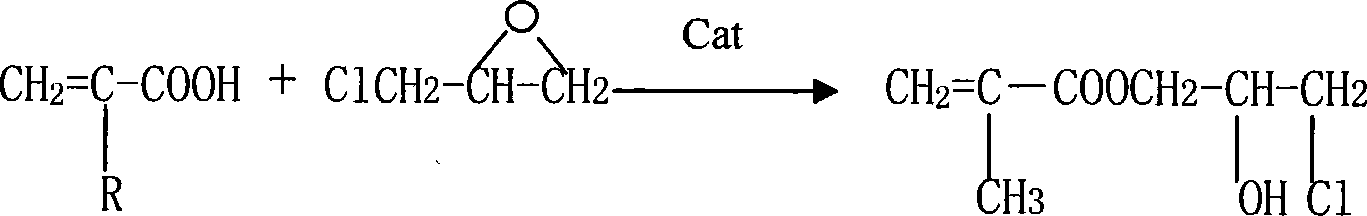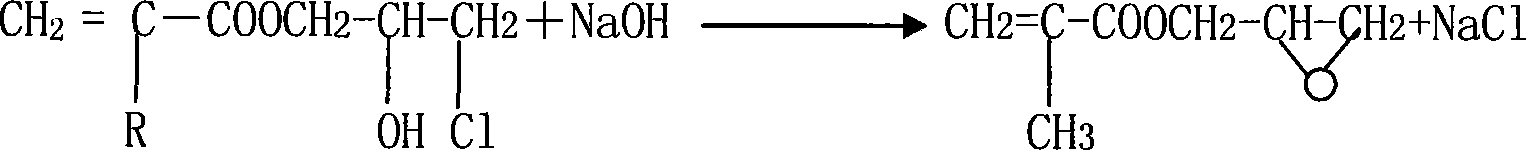Synthesis method for (methyl)glycidyl acrylate
A technology of glycidyl ester and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of long reaction steps, large amount of epichlorohydrin, and complicated treatment, and achieve the effects of mild reaction conditions, less environmental pollution, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] Add 117g of methacrylic acid (MAA), 1.7g of hexamethylenetetramine, 0.4g of p-hydroxyanisole into a 500mL four-neck flask with a mechanical stirrer, turn on the stirrer, raise the temperature to 80°C, and add 175g of For epichlorohydrin (ECH), control the reaction temperature at 80-90°C. After the epichlorohydrin has been added dropwise for 6 hours, keep it warm for 1 hour; when the reaction is over, cool the reaction product below 40°C and start to add 32% by weight of hydrogen dropwise. 190 grams of sodium oxide aqueous solution, the reaction temperature is controlled at 35-45 ° C, after 3 hours of dropwise addition, the reaction is kept for 1 hour; 129.1 g of glycidyl methacrylate product is obtained, and the epoxy value is 0.481 eq / 100 g.
example 2
[0027] Add 117g of methacrylic acid, 1.7g of hexamethylenetetramine, and 0.2g of p-hydroxyanisole to a 500mL four-neck flask with a mechanical stirrer, turn on the stirrer, raise the temperature to 80°C, and add 132g of epoxy chloride dropwise Propane (ECH), control the reaction temperature at 60-70°C, after adding epichlorohydrin 6h dropwise, keep warm for 1h reaction; after the reaction is finished, cool the reaction product to below 40°C, and start to drop 32% by weight of sodium hydroxide aqueous solution 190g, control the reaction temperature at 35-45°C, after 3h of dropwise addition, keep warm for 1h to obtain 50.6g of glycidyl methacrylate product with an epoxy value of 0.503eq / 100g.
example 3
[0029] Add 117g of methacrylic acid, 1.25g of quinoline, and 0.4g of p-hydroxyanisole into a 500mL four-necked flask with a mechanical stirrer, turn on the stirrer, raise the temperature to 80°C, and add dropwise 155g of epichlorohydrin (ECH) , control reaction temperature 80~90 ℃, after epichlorohydrin 5h dropwise, insulation reaction 1h; Reaction finishes, reaction product is cooled to below 40 ℃, starts to drip 120 grams of 50% by weight sodium hydroxide aqueous solution, control The reaction temperature was 35-45°C. After 3 hours of dropwise addition, the reaction was kept for 2 hours to obtain 129g of glycidyl methacrylate product, and the epoxy value was 0.492eq / 100g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com