Method for expelling nitrous acid alkyl ester and nitrogen oxide gas from the discharged gas
A technology of alkyl nitrite and nitrogen oxides, applied in chemical instruments and methods, separation methods, air quality improvement and other directions, to achieve the effects of reducing costs, eliminating pollution, and simple process technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
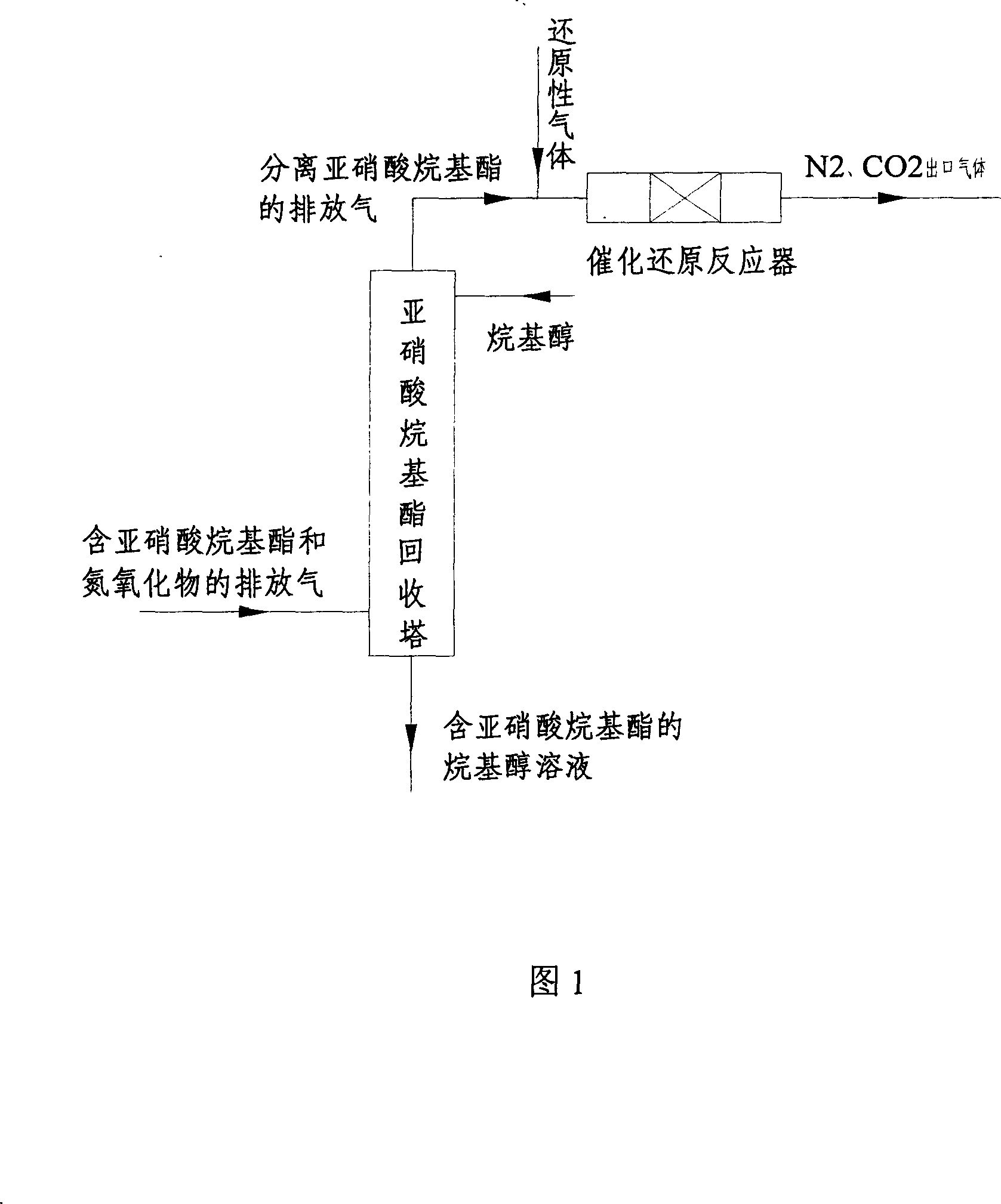
[0020] According to flow chart 1, will contain CO-12.0V%, nitrogen oxide-0.5V% and CH 3 ONO-11.0V% synthesis of dimethyl oxalate reaction tail gas 40L / hr, and 121.2ml / hr of -10 ℃ methanol convective contact with the introduction from the top of the tower. The methanol solution after absorbing methyl nitrite is taken out from the bottom of the tower and returned to the main reaction system; the gas from the top of the tower passes through the bed layer equipped with a supported catalyst, and is heated at 250-300°C and the gas hourly space velocity (GHSV) is 30000-40000h -1 The catalytic reduction reaction is carried out under the following conditions, and the gas after the reaction is analyzed by gas chromatography: CO-11.0V%, nitrogen oxides-0.00V%, and the rest of the gas is N 2 . The reacted gas is discharged into the atmosphere after being burned by a torch.
Embodiment 2
[0022] According to flow chart 1, will contain CO-2.30V%, nitrogen oxide-1.0V% and CH 3 CH 2 ONO-10.0V% synthetic diethyl oxalate reaction tail gas 50L / hr, and 138ml / hr methanol convection contact at -3°C introduced from the top of the tower. The methanol solution after absorbing ethyl nitrite is taken out from the bottom of the tower and returned to the main reaction system; the gas from the top of the tower passes through the bed layer equipped with a supported catalyst, at 250~350℃, GHSV 30000~40000h -1 The catalytic reduction reaction is carried out under the following conditions, and the gas after the reaction is analyzed by gas chromatography: CO-1.3V%, nitrogen oxides-0.00V%, and the rest of the gas is N 2 . The reacted gas is discharged into the atmosphere after being burned by a torch.
Embodiment 3~8
[0024] According to flow chart 1, the tail gas of Examples 3-8 is processed, and the experimental results are shown in Table 1.
[0025] Table 1
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com