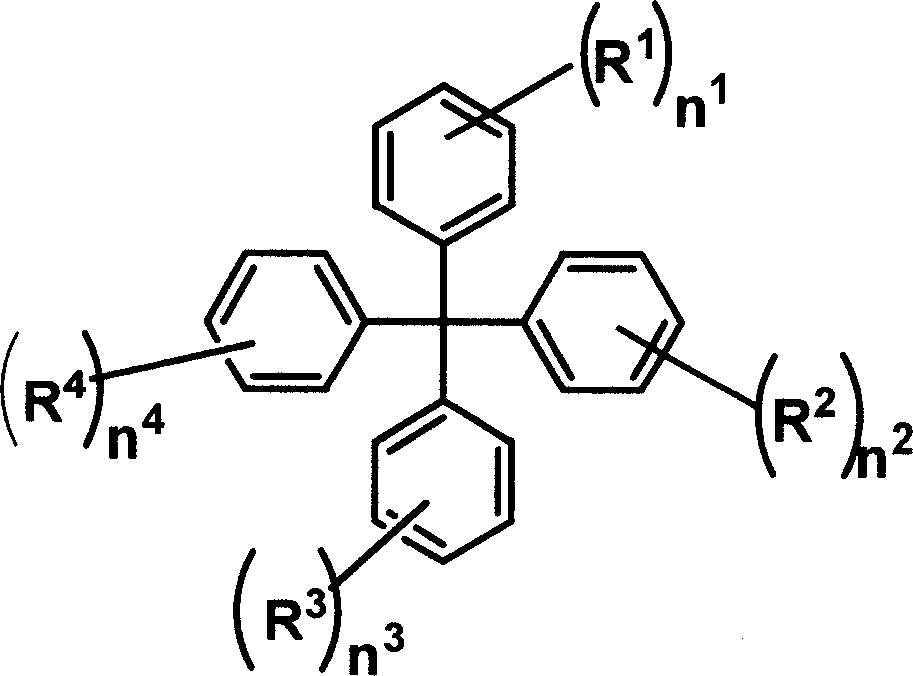
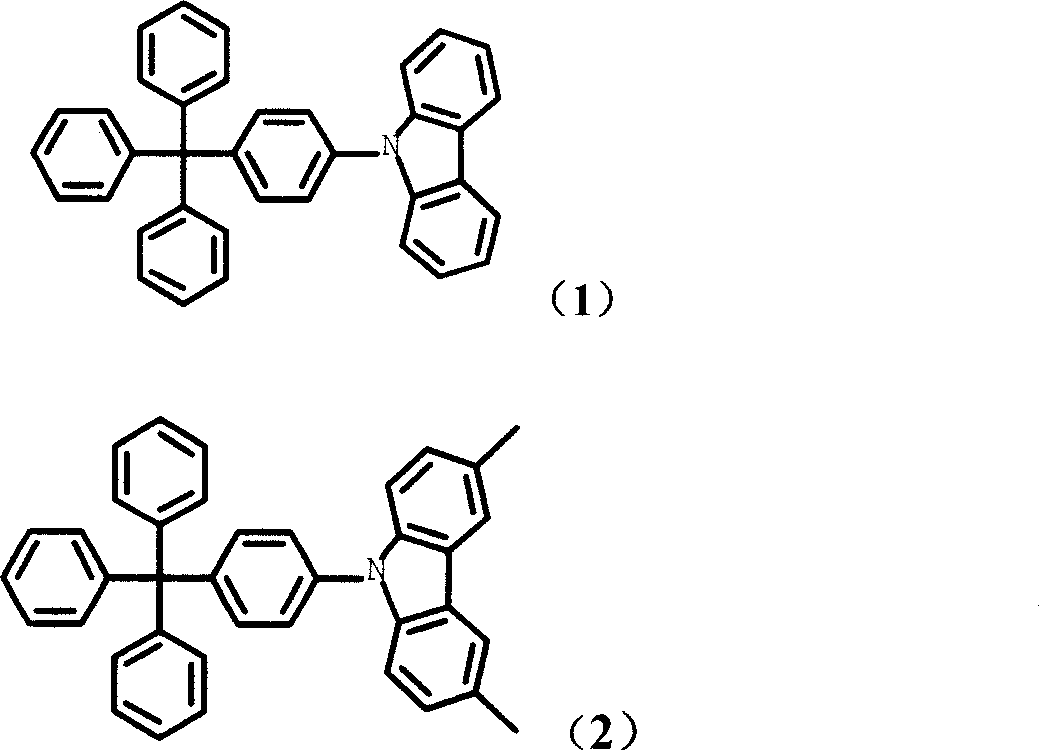
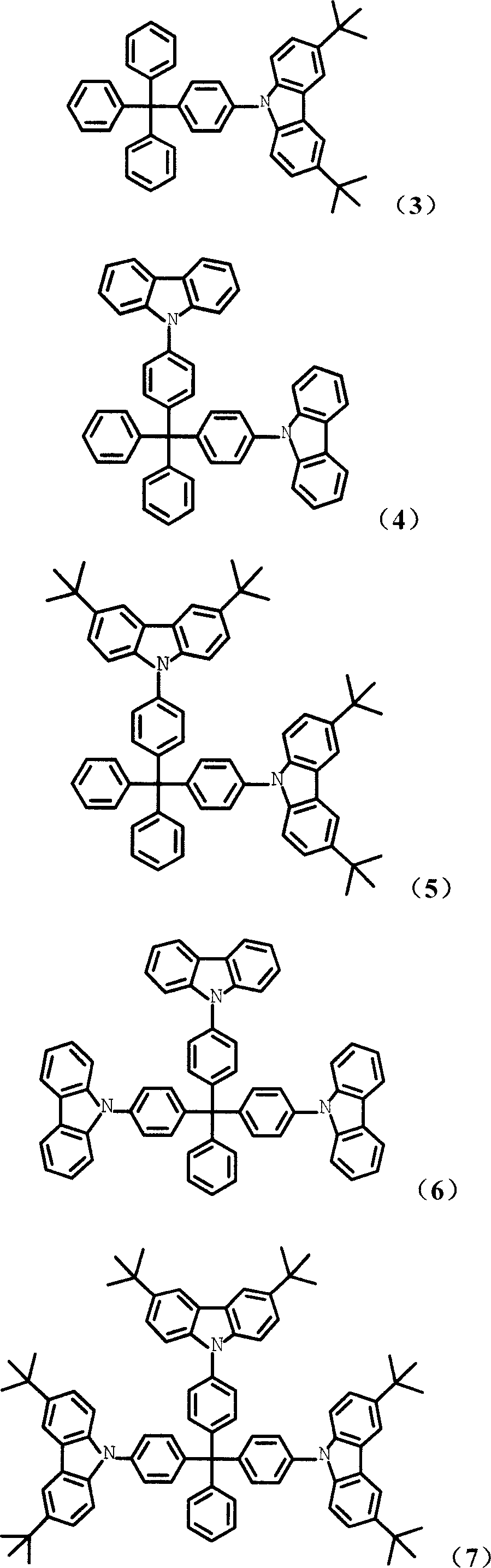
Tetrabenzene methane derivative and its application
A technology of tetraphenylmethane and derivatives, used in triarylmethane dyes, methine/polymethine dyes, electrical components, etc., can solve the problems of short life and low efficiency, and achieve improved life, high brightness, and improved efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the synthesis of compound (1)
[0048] Add 0.1mol copper powder, 0.015mol 18-crown-6, 0.2mol potassium carbonate in the flask, and pass through nitrogen gas, add o-dichlorobenzene, 0.1mol 4-iodotetraphenylmethane and 0.11 mol carbazole. Heating to 180°C-200°C, reacting for 16-48 hours. After the reaction was completed, it was filtered while hot, and the yield was 62%. Mass spectrum: m / e, 485; elemental analysis: experimentally determined C: 91.49%, H: 5.66%, N: 2.75%; theoretical values: C: 91.51%, H: 5.60%, N: 2.88%.
Embodiment 2
[0049] Embodiment 2: the synthesis of compound (3)
[0050] Synthesized according to the method of compound (1), replacing carbazole with 3,6-di-tert-butylcarbazole, and the yield is 59%. Mass spectrum: m / e, 597; elemental analysis: experimentally determined C: 90.35%, H: 7.33%, N: 2.32%; theoretical values: C: 90.41%, H: 7.25%, N: 2.34%.
Embodiment 3
[0051] Embodiment 3: the synthesis of compound (4)
[0052] Synthesized according to the method of compound (1), the feeding amount of carbazole is 0.21mol, and 4,4'-diiodotetraphenylmethane is used instead of 4-iodotetraphenylmethane, and the yield is 66%. Mass spectrum: m / e, 650; elemental analysis: experimentally determined C: 90.40%, H: 5.31%, N: 4.25%; theoretical values: C: 90.43%, H: 5.27%, N: 4.30%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com