Alprostadil and vitamin F millimicroball composite medicine and its preparation method
An alprostadil and vitamin technology, applied in the field of nanosphere combination medicine and preparation thereof, can solve the problems of unacceptable to patients, low bioavailability, short elimination half-life, etc., and achieves reduced vascular irritation, excellent curative effect, and good targeting effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The formula adopted in the preparation of blank nanospheres in this embodiment is as follows:
[0053] Gelatin mixed with casein and human gamma globulin in any proportion 200g
[0054] Hydroxypropyl-β-cyclodextrin 500g
[0055] Pluronic F68 15g
[0056] Soybean oil and ethyl acetate weight ratio 3:1 mixed oil 1000g
[0057] Ethanol 8000g
[0058] Add water for injection to 1000ml
[0059] The method steps for preparing blank nanospheres are as follows:
[0060] (1) Dissolve the mixture of the amount of gelatin, casein and human gamma globulin in any proportion in the amount of water for injection, dissolve completely under stirring, maintain the solution temperature at 55 ± ℃, and add the amount of cyclodextrin, Pluronic F68 and PEG6000 mixture, completely dissolved;
[0061] (2) Cool the solution prepared in step (1) to room temperature, add it to an equal volume of vegetable oil and ethyl acetate mixture, emulsify, and continue cooling the formed emulsion to 5-...
Embodiment 2
[0085] Preparation of blank nanosphere recipe:
[0086] Gelatin mixed with casein and human gamma globulin in any proportion 300g
[0087] γ-cyclodextrin 200g
[0088] Mixture of Pluronic F68 and polyethylene glycol 6000 in any proportion 30g
[0089] The mixture of peanut oil and ethyl acetate 3:1 weight ratio 2000g
[0090] Ethanol 10000g
[0091] Water for injection 2000g
[0092] The preparation steps and method are the same as in Example 1.
[0093] Preparation method of nanosphere carrier drug:
[0094] Blank nanospheres prepared above 150g
[0095] Alprostadil or vitamin F mixture in any proportion 5g
[0096] Tween80 4g
[0097] Vitamin C 20g
[0098] The mixture of N-(2-mercaptopropionyl)-glycine and cysteine with a weight ratio of 2-4:1 40g
[0099] Add water for injection to 2000ml
[0100] The preparation steps and method are the same as in Example 1.
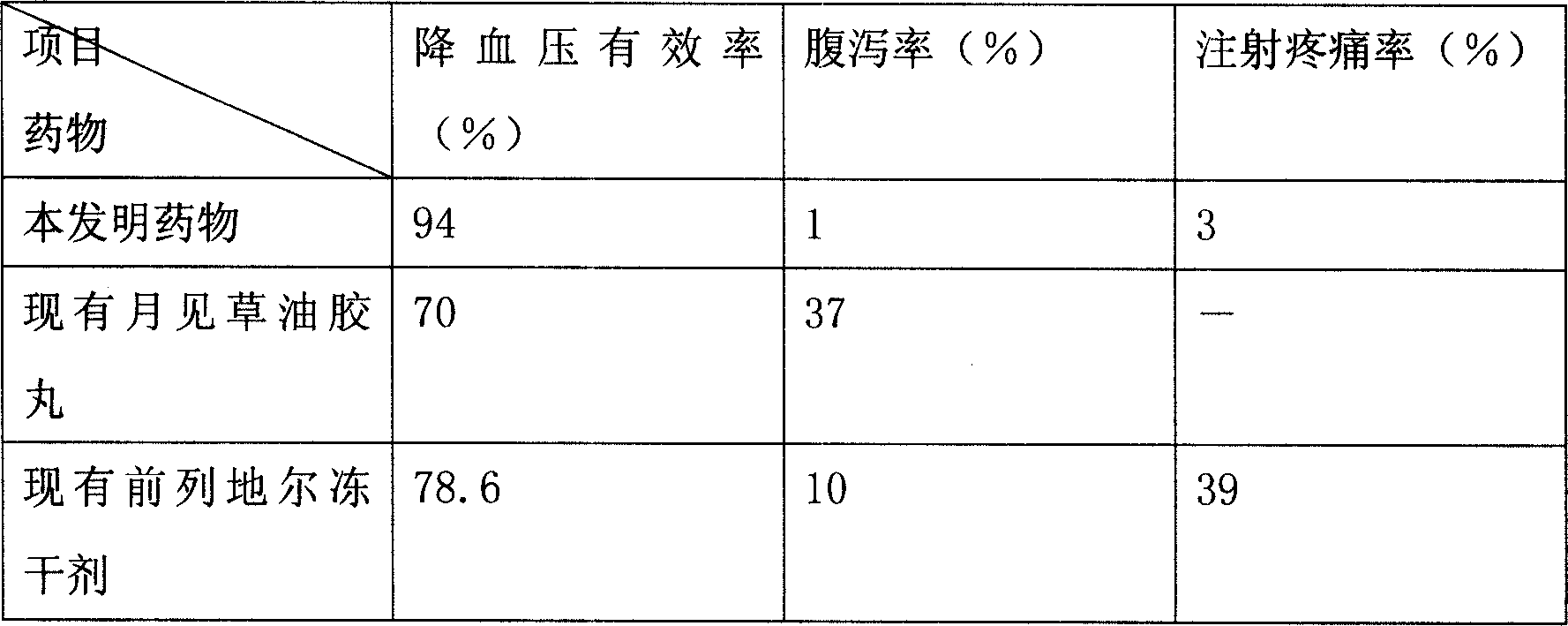
[0101] Drug efficacy verification test:
[0102]A rat model of renovascular hypertension was used....
Embodiment 3
[0104] Preparation of blank nanosphere recipe:
[0105] Gelatin mixed with casein and human gamma globulin in any proportion 260g
[0106] Mixture of hydroxypropyl-β-cyclodextrin and γ-cyclodextrin in any proportion 140g
[0107] Mixture of Pluronic F68 and polyethylene glycol 6000 in any proportion 22g
[0108] The mixture of soybean oil, peanut oil and ethyl acetate in a weight ratio of 3:1 1680g
[0109] Ethanol 7000g
[0110] Water for injection 1660g
[0111] The preparation steps and method are the same as in Example 1.
[0112] Preparation method of nanosphere carrier drug:
[0113] Blank nanospheres prepared above 130g
[0114] Alprostadil or vitamin F mixture in any proportion 3g
[0115] Tween80 3g
[0116] Vitamin C 16g
[0117] The mixture of N-(2-mercaptopropionyl)-glycine and cysteine with a weight ratio of 2-4:1 32g
[0118] Add water for injection to 1600ml
[0119] The preparation steps and method are the same as in Example 1.
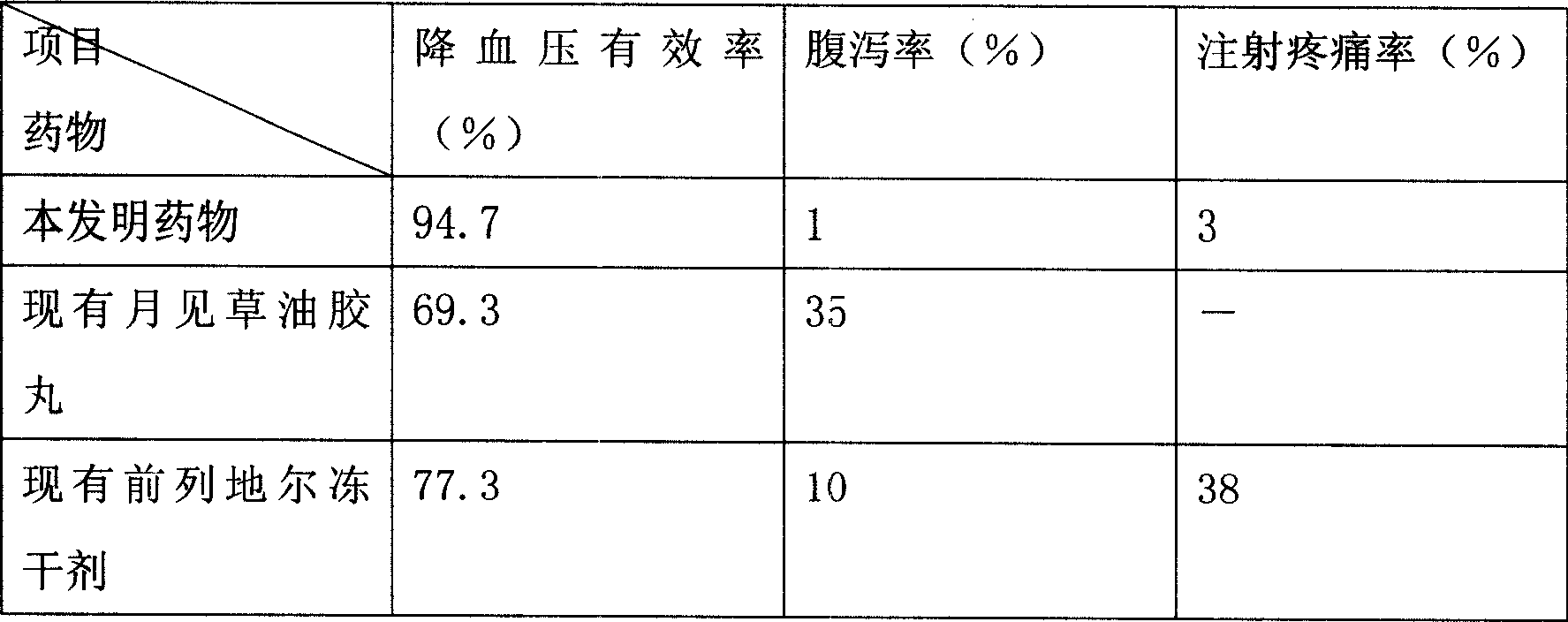
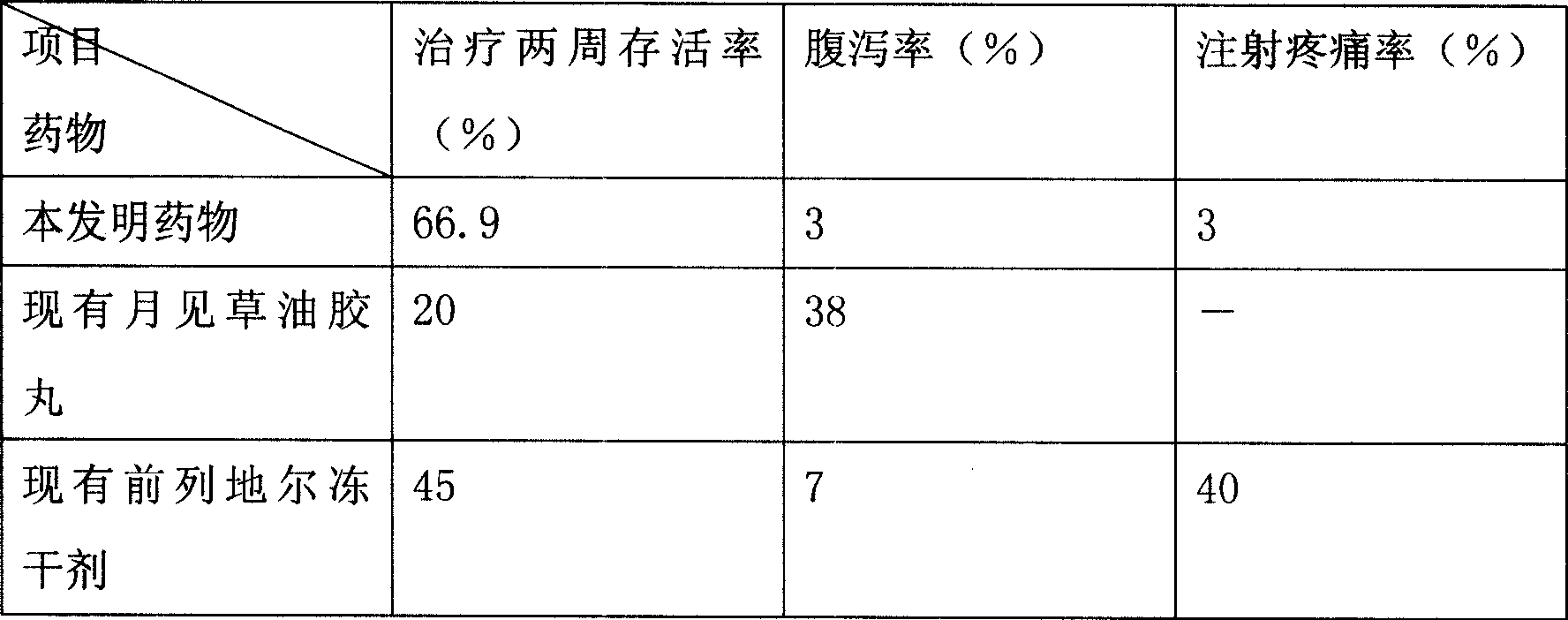
[0120] Drug effic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com