A kind of ceftiofur crystal suspension injection and preparation method thereof
A technology of ceftiofur crystals and suspension injection, which is applied in liquid delivery, pharmaceutical formula, emulsion delivery, etc. It can solve the problems of waste of human and financial resources in farms, animal stress response, short action time, etc., and achieve improved mixing. Suspension adhesion effect, uniform mixing, long half-life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
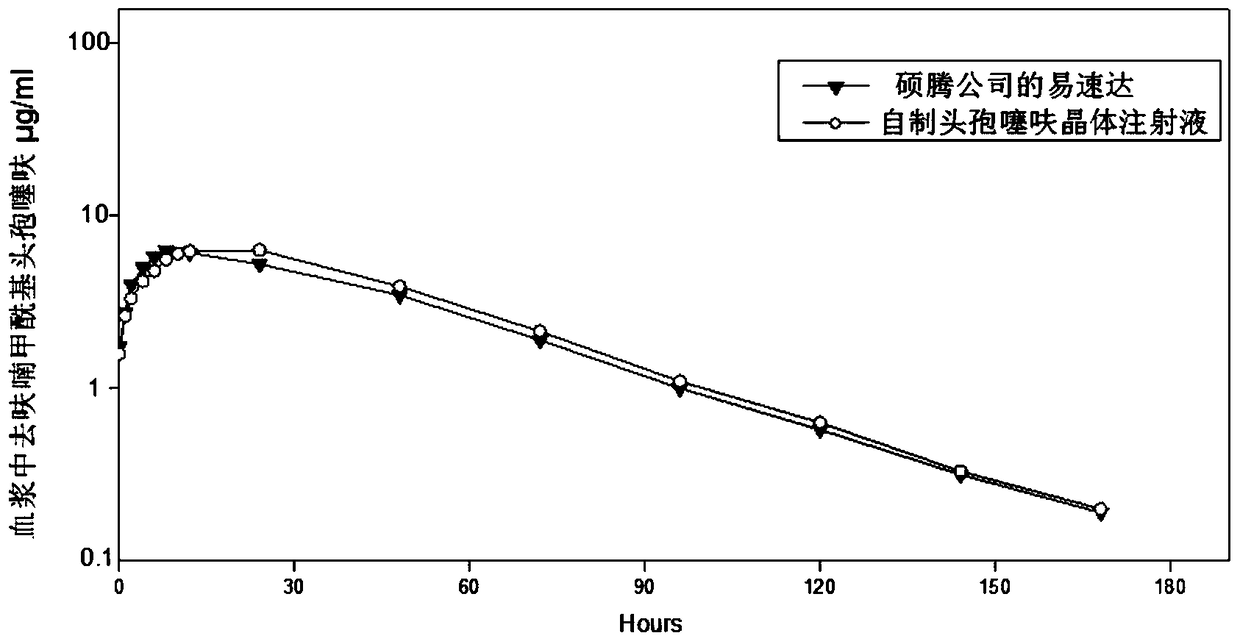
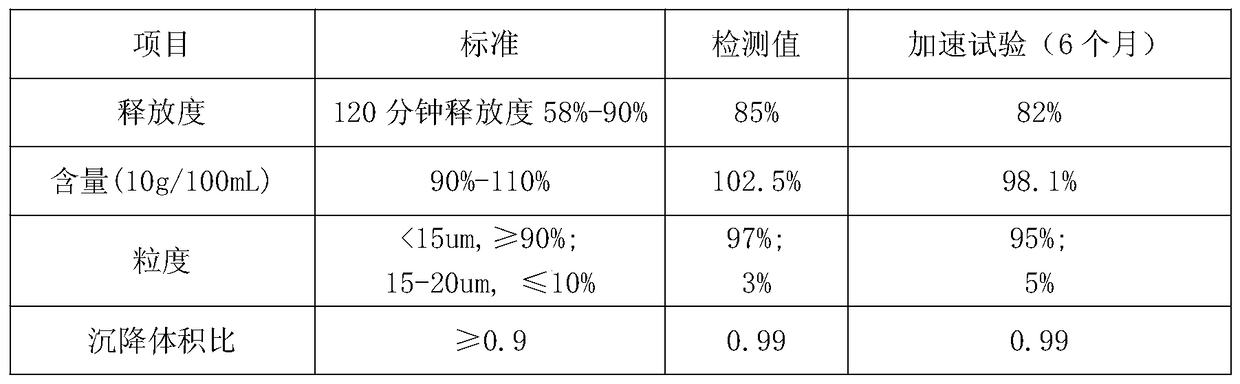
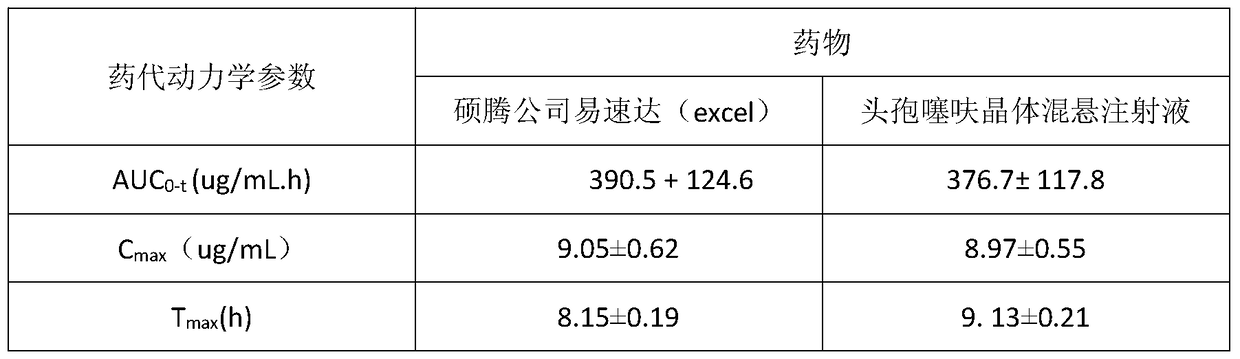
Image
Examples
Embodiment 1
[0029] 10% ceftiofur crystal suspension injection formula 1:
[0030] raw material
Feeding
content
Ceftiofur crystals
537.6g
10% (W / V)
Povidone K-17
250g
2% (W / V)
polyethylene glycol 400
1.5L
30% (V / V)
50mL
1% (V / V)
3.06L
[0031] Preparation:
[0032] 1) dissolving povidone K-17 in polyethylene glycol 400, adding soybean oil to obtain an injection vehicle;
[0033] 2) adding benzyl alcohol to the injection solvent in step 1;
[0034] 3) Under nitrogen protection, sterilize the mixed solvent in step 2 at 135° C. for 5 hours, and place it at room temperature for later use;
[0035] 4) Under the protection of nitrogen, add ceftiofur crystals to the mixed solvent, and use a high-speed shear disperser to stir for 30 minutes under the condition of 1200r / min, and mix well;
[0036] 5) Go through a colloid mill to obtain ceftiofur crystal suspension injection.
...
Embodiment 2
[0057] 10% Ceftiofur Crystalline Suspension Injection Formula II:
[0058] raw material
Feeding
proportion
Ceftiofur crystals
537.6g
10% (W / V)
Povidone K-12
250g
2% (W / V)
0.5L
10% (V / V)
25mL
0.5% (V / V)
4.08L
[0059] Preparation:
[0060] 1) Dissolving povidone K-12 in polyethylene glycol 300, adding olive oil, to obtain an injection vehicle;
[0061] 2) Add chlorobutanol to the injection vehicle in step 1;
[0062] 3) Under argon protection, sterilize the mixed solvent in step 2 at 140° C. for 3 hours, and place it at room temperature for later use;
[0063] 4) Under the protection of argon, add ceftiofur crystals to the mixed solvent, and use a high-speed shear disperser to stir for 15 minutes under the condition of 2000r / min, and mix well;
[0064] 5) Obtaining ceftiofur crystal suspension injection through colloid milling.
Embodiment 3
[0066] 15% Ceftiofur Crystalline Suspension Injection Formula:
[0067] raw material
Feeding
proportion
Ceftiofur crystals
805.4g
15% (W / V)
Povidone K-17
625g
5% (W / V)
0.5L
10% (V / V)
50mL
1% (V / V)
4.74L
[0068] Preparation:
[0069] 1) Dissolving povidone K-17 in polyethylene glycol 400, adding ethyl oleate to obtain an injection vehicle;
[0070] 2) Add chlorobutanol to the injection vehicle in step 1;
[0071] 3) Under nitrogen protection, sterilize the mixed solvent in step 2 at 135° C. for 5 hours, and place it at room temperature for later use;
[0072] 4) Under the protection of nitrogen, add ceftiofur crystals to the mixed solvent, and use a high-speed shear disperser to stir for 15 minutes under the condition of 1500r / min, and mix well;
[0073] 5) Obtaining ceftiofur crystal suspension injection through colloid milling.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com