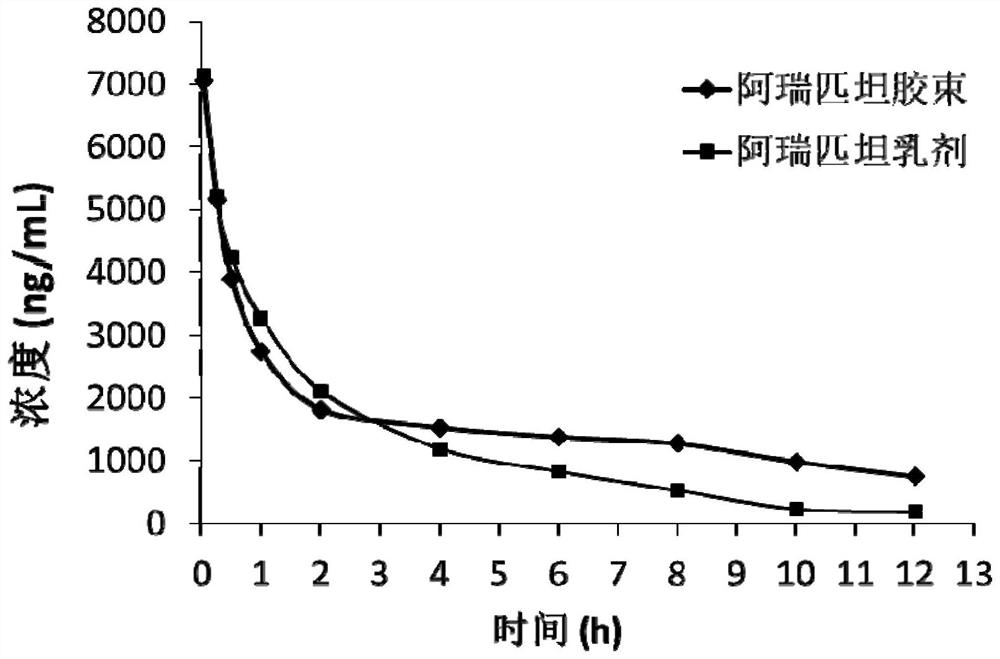
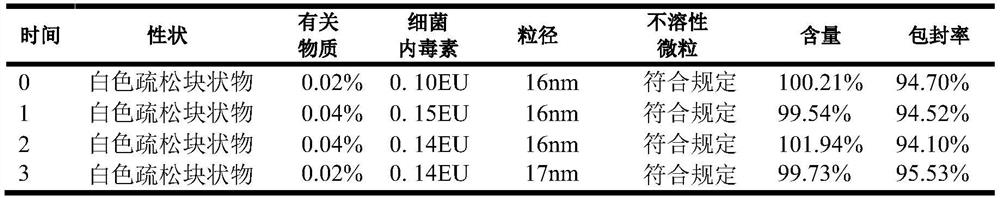
Aprepitant micellar sterile freeze-dried preparation for intravenous injection and preparation method thereof
A freeze-dried preparation, aprepitant technology, applied in the field of medicine, can solve the problems of limited clinical use, systemic allergic reactions, low oral bioavailability, etc., and achieve the effects of improving bioavailability and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] In a round-bottomed flask, 75 mg of cultured phosphatidylethanolamine and 15 mg of aprepitant were dissolved in 2 mL of ethanol, and the ethanol was evaporated using a rotary evaporator in a water bath at 50 °C, so that the polymer and the drug formed a A uniform film was then hydrated with 5 mL of deionized water and allowed to stand for 30 min to fully self-assemble to form micelles. An appropriate amount of sodium hydroxide solution was added to adjust the pH to 7.5, and 0.9 g of lactose was added. The filter membrane was filtered and sterilized, and the filtrate was freeze-dried for 72 hours to remove water, and finally a sterile freeze-dried preparation of aprepitant micelle was prepared.
[0046] Determination of relevant properties of aprepitant micellar solution:
[0047] Particle size: determined by dynamic light scattering method, it is 17nm;
[0048] Drug encapsulation efficiency: determined by high performance liquid chromatography, it is 93.8%;
[0049] p...
Embodiment 2
[0051] In a round-bottomed flask, 150 mg of cultured phosphatidylethanolamine and 15 mg of aprepitant were dissolved in 3 mL of ethanol, and the ethanol was evaporated using a rotary evaporator in a water bath at 50 °C, so that the polymer and the drug formed a A uniform film was then hydrated with 10 mL of deionized water and allowed to stand for 30 minutes to fully self-assemble to form micelles. An appropriate amount of sodium hydroxide solution was added to adjust the pH to 7.5, and 1.65 g of lactose was added. The filter membrane was filtered and sterilized, and the filtrate was freeze-dried for 72 hours to remove water, and finally a sterile freeze-dried preparation of aprepitant micelle was prepared.
[0052] Determination of relevant properties of aprepitant micellar solution:
[0053] Particle size: determined by dynamic light scattering method, it is 25nm;
[0054] Drug encapsulation efficiency: determined by high performance liquid chromatography, it is 94.5%;
[...
Embodiment 3
[0057] In a round-bottomed flask, 300 mg of cultured phosphatidylethanolamine and 15 mg of aprepitant were dissolved in 5 mL of ethanol, and the ethanol was evaporated using a rotary evaporator in a water bath at 50 °C, so that the polymer and the drug formed a A uniform film was then hydrated with 15 mL of deionized water and allowed to stand for 30 minutes to fully self-assemble to form micelles. An appropriate amount of sodium hydroxide solution was added to adjust the pH to 7.5, and 3.15 g of lactose was added. The filter membrane was filtered and sterilized, and the filtrate was freeze-dried for 72 hours to remove water, and finally a sterile freeze-dried preparation of aprepitant micelle was prepared.
[0058] Determination of relevant properties of aprepitant micellar solution:
[0059] Particle size: determined by dynamic light scattering method, it is 28nm;
[0060] Drug encapsulation efficiency: determined by high performance liquid chromatography, it is 95.3%;
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com