Quantitative nano aerosol and method of manufacturing the same
An aerosol and nano-technology, applied in the field of nano-aerosol, can solve the problems of poor dispersion of drug particles and low lung delivery efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] Aerosol preparation
[0077] According to the requirements of conventional treatment and the type of medicine, the amount of chemical medicine or biomolecule contained in each aerosol is usually 0.01mg-2mg, for example, each dose of albuterol is 100-200μg. A certain amount of the mixture of nano drug particles and surfactant is taken and placed in an aerosol can. Add a small amount of low-alkane such as n-heptane to dissolve the surfactant and disperse the nano drug particles, and then seal with a quantitative valve (100 μl). Then inject the fluoroalkane HFC134a to obtain a uniformly dispersed and extremely stable suspension (Figure 4A). After standing for four months, neither sediment nor floating matter appeared (Fig. 4B). Therefore, very stable aerosols can be successfully prepared using nano-drug particles, and their good dispersion state shows their excellent ability in pulmonary drug delivery.
[0078] Testing for Pulmonary Drug Delivery
[0079] Pulmonary dru...
Embodiment 1
[0083] Lecithin is dissolved in isopropanol to make a surfactant solution (mass ratio is lecithin: isopropanol=1:3). Add 56.3mL of surfactant solution into 43.5mL of isooctane, oscillate evenly, and let stand for 30 minutes. Then add 23.8mL of 15% (mass percentage) salbutamol aqueous solution, shake vigorously, mix evenly, make it become microemulsion.
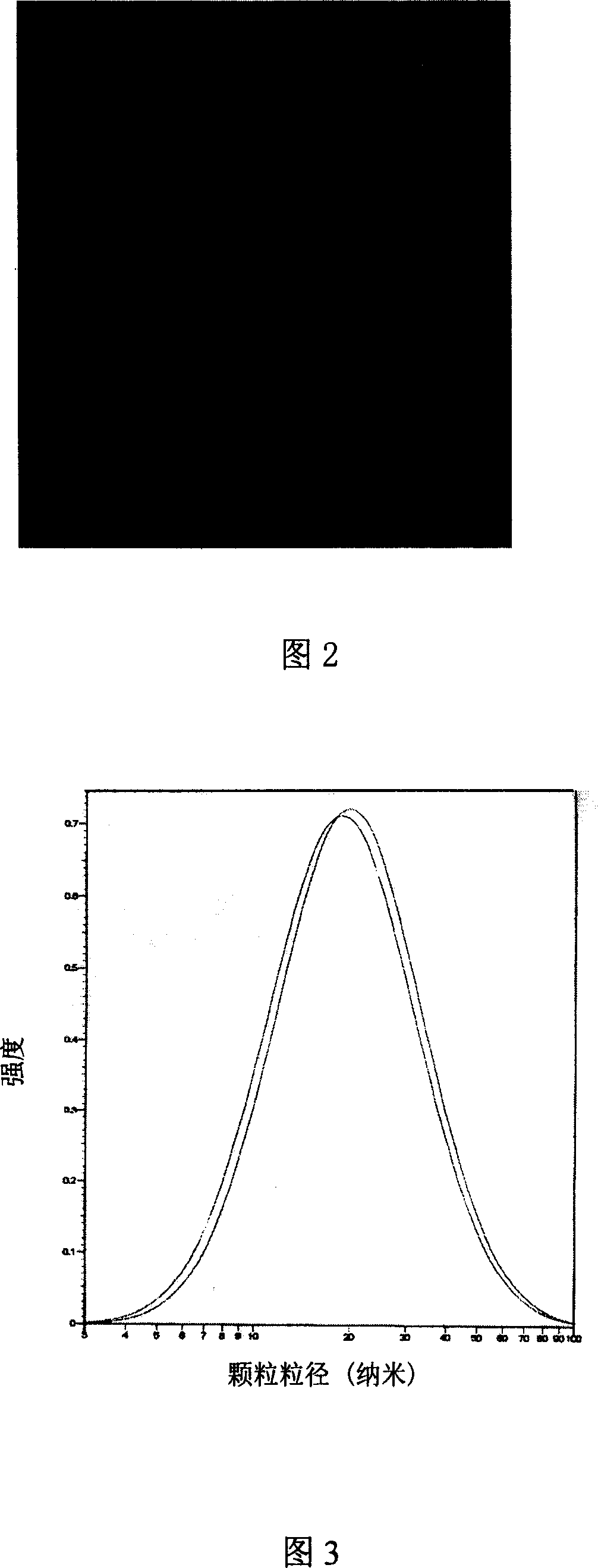
[0084] The standard operating procedure for the preparation of albuterol nanoparticles is as follows. The spray dryer sprays and dries the above-mentioned microemulsion under standard working conditions. After completion, use n-heptane to dissolve and recover the dry matter deposited in the spray chamber, cyclone centrifuge and collector. Dry naturally at room temperature, remove the n-heptane, and obtain a dry mixture containing nano albuterol particles, the average particle diameter of which is close to 20nm.
[0085] Take 30 mg of the mixture (containing 7.5 mg of drug) and put it into an aerosol can, and add 1 mL of n-h...
Embodiment 2
[0087] In Example 1, 30 mg (containing 7.5 mg of drug) was weighed from the mixture containing nano-medicine particles prepared by spray drying and put into an aerosol can, and 1 mL of n-pentane was injected to uniformly disperse the nano-medicine particles in the in. Seal it with a 100 μL metering valve, and then inject 6.5 mL of propellant HFC134a to obtain an aerosol in a fluid in which nano drug particles are uniformly dispersed. This 100μg / spray nano-aerosol can maintain a very stable dispersion state. The microparticle ratio (particle size<5.8 μm) was determined to be 61% by lung model.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com