Quick disintegration tablet and method of producing the same
A manufacturing method and technology for rapidly disintegrating tablets, applied in the field of rapidly disintegrating tablets and their manufacturing, can solve the problems of tablet adhesion, tablet fragility, melting and the like, and achieve the effects of sufficient tablet strength, easy taking and high strength.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
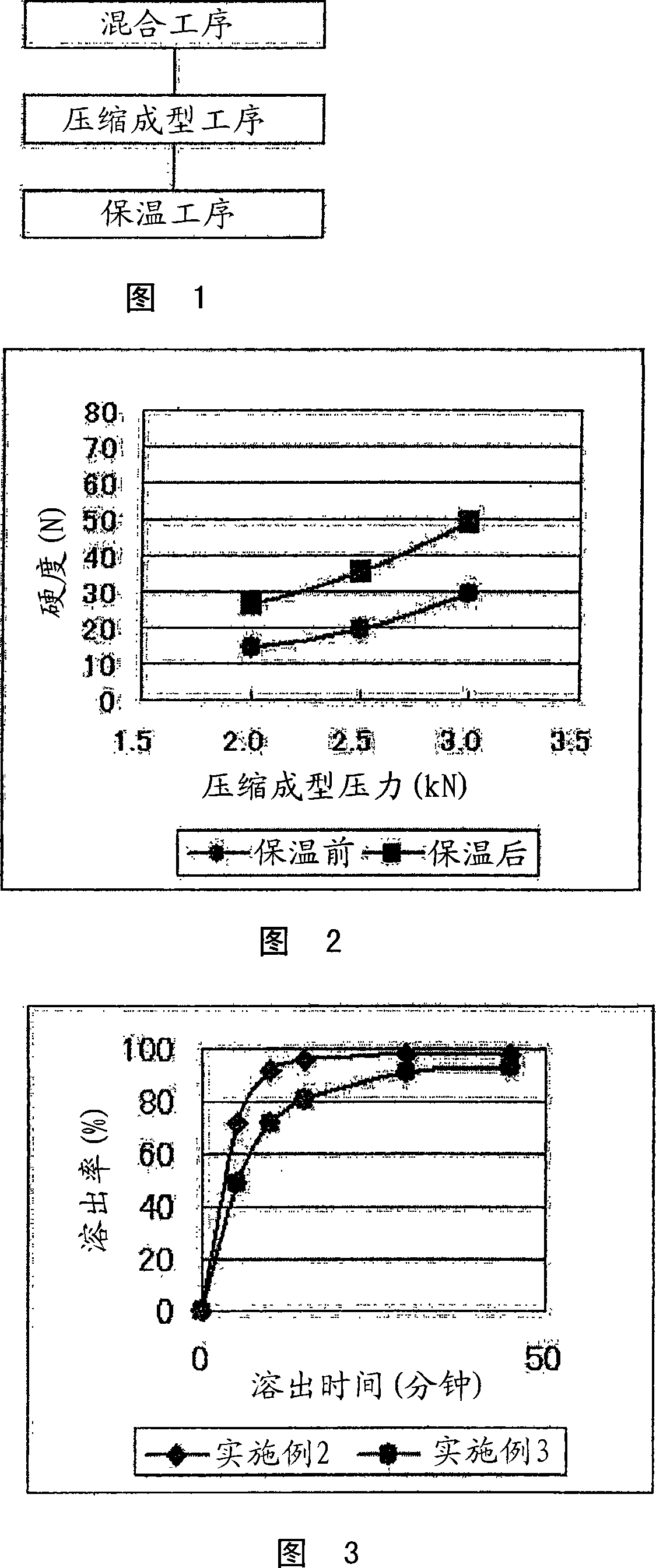
[0100] Using a mechanical mixer (manufactured by Okada Seiko Co., Ltd.), 115.36 g of crystalline cellulose (trade name: ABISEL PH301, manufactured by Asahi Kasei Industries, Ltd., hereinafter referred to as "ABISEL PH301") and 173.04 g of mannose were mixed as the above-mentioned additive at 800 rpm. Alcohol (trade name: Parteck M200, manufactured by Merck) and 11.6 g of aminoalkyl methacrylate copolymer E (trade name: オイドラギッッド (registered trademark) EPO, Dagusa Co., Ltd.) as the above-mentioned acrylic copolymer for 3 minutes. Using the Kikusui small high-speed rotary tablet press (VIRGO 0512SS2AZ)-external lubricant spray system (ELS-P1 type I) to target the weight of the obtained mixture at 180 mg, under the conditions of compression molding pressure 2.0kN, 2.5kN, 2.9kN , respectively compression molding the above mixture. At this time, a die ring and a punch having an inner diameter of 8 mm were used, and magnesium stearate was used as an external lubricant to obtain a com...
Embodiment 2
[0107] 0.35 g of amlodipine besylate (manufactured by Dr. Reddy's Company) as the above-mentioned drug, and 6.9 g of crystalline cellulose as the above-mentioned additive (trade name: Abicel PH101, manufactured by Asahi Kasei Industries, Ltd., hereinafter) were thoroughly mixed using a polyethylene bag. called "ABISEL PH101"), 10.4 g of mannitol (manufactured by Towa Chemical Industry Co., Ltd.), and 0.35 g of aminoalkyl methacrylate copolymer E (trade name: オイドラギッド (registered trademark) EPO , Dagusa Company) to obtain the mixture. Next, the resulting mixture was compression-molded using Shimadzu Autograph AGS-1000D under a compression molding pressure of 2.9 kN to obtain a compression-molded product with a weight of 180 mg and a diameter of 8 mm. For compression molding, apply magnesium stearate (NOF Co., Ltd.) to the ring and punch.
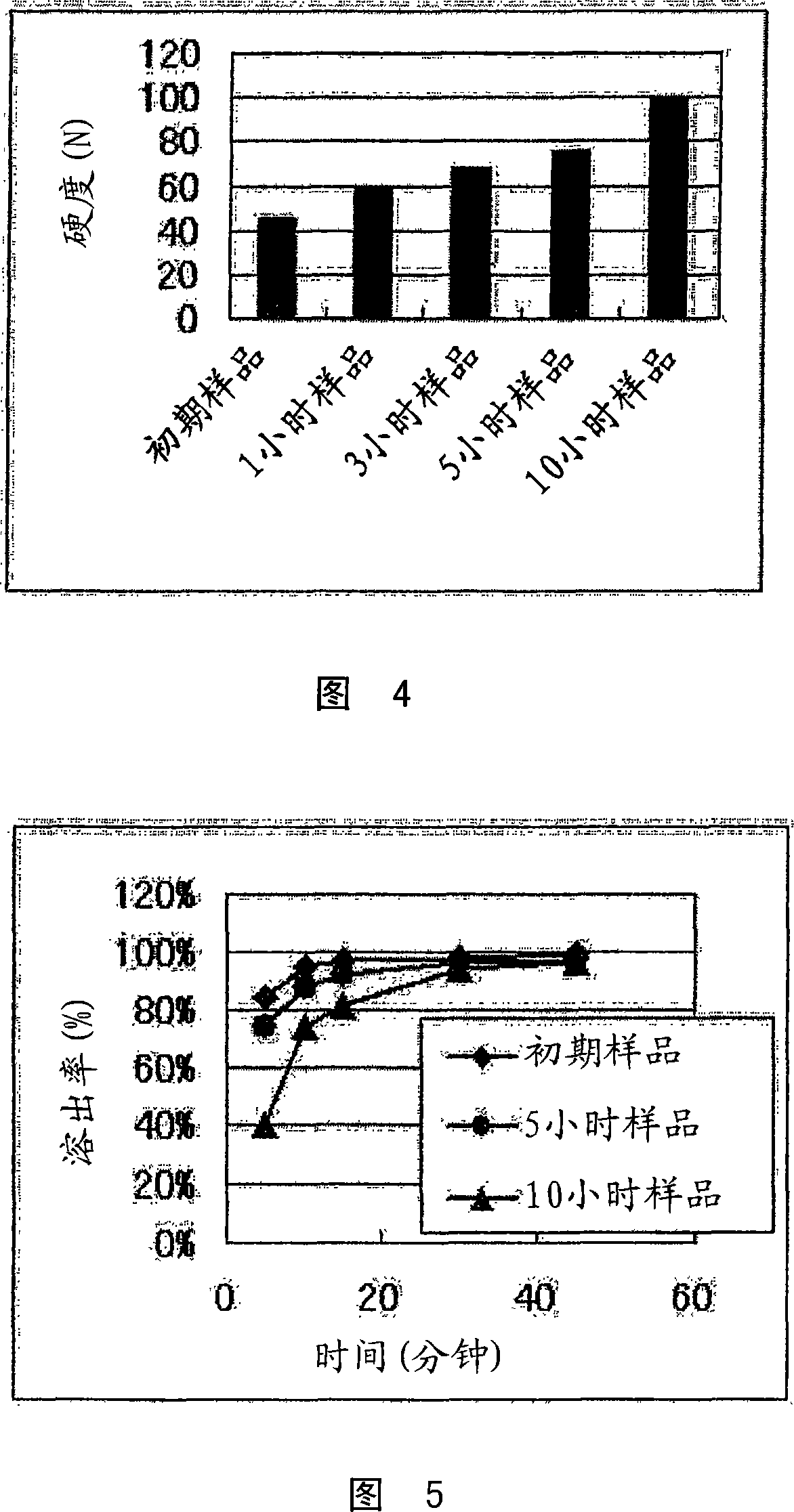
[0108] The compression-molded product was kept warm in a constant temperature bath (80° C. holding temperature) for 10 hours, and then left ...
Embodiment 3
[0119] 0.054 g of amlodipine besylate (manufactured by Dr. Reddy's Company) as the above-mentioned drug, 1.13 g of Abicel PH101 (manufactured by Asahi Kasei Industries, Ltd.) and 1.70 g of mannitol (manufactured by Towa Kasei Kogyo Co., Ltd.) and 0.114 g of aminoalkyl methacrylate copolymer E (trade name: オイドラギッド (registered trademark) EPO, Dagusa Corporation) as the above-mentioned acrylic copolymer to obtain a mixture.
[0120] Next, the obtained mixture was compression-molded using Shimadzu Autograph AGS-1000D under a compression molding pressure of 2.9 kN to obtain a compression-molded product with a weight of 180 mg and a diameter of 8 mm. For compression molding, apply magnesium stearate (NOF Co., Ltd.) to the ring and punch.
[0121] The compression-molded product was kept warm in a constant temperature bath (80° C. holding temperature) for 10 hours, and then left to cool to room temperature to obtain the above-mentioned rapidly disintegrating tablet (orally rapidly dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com