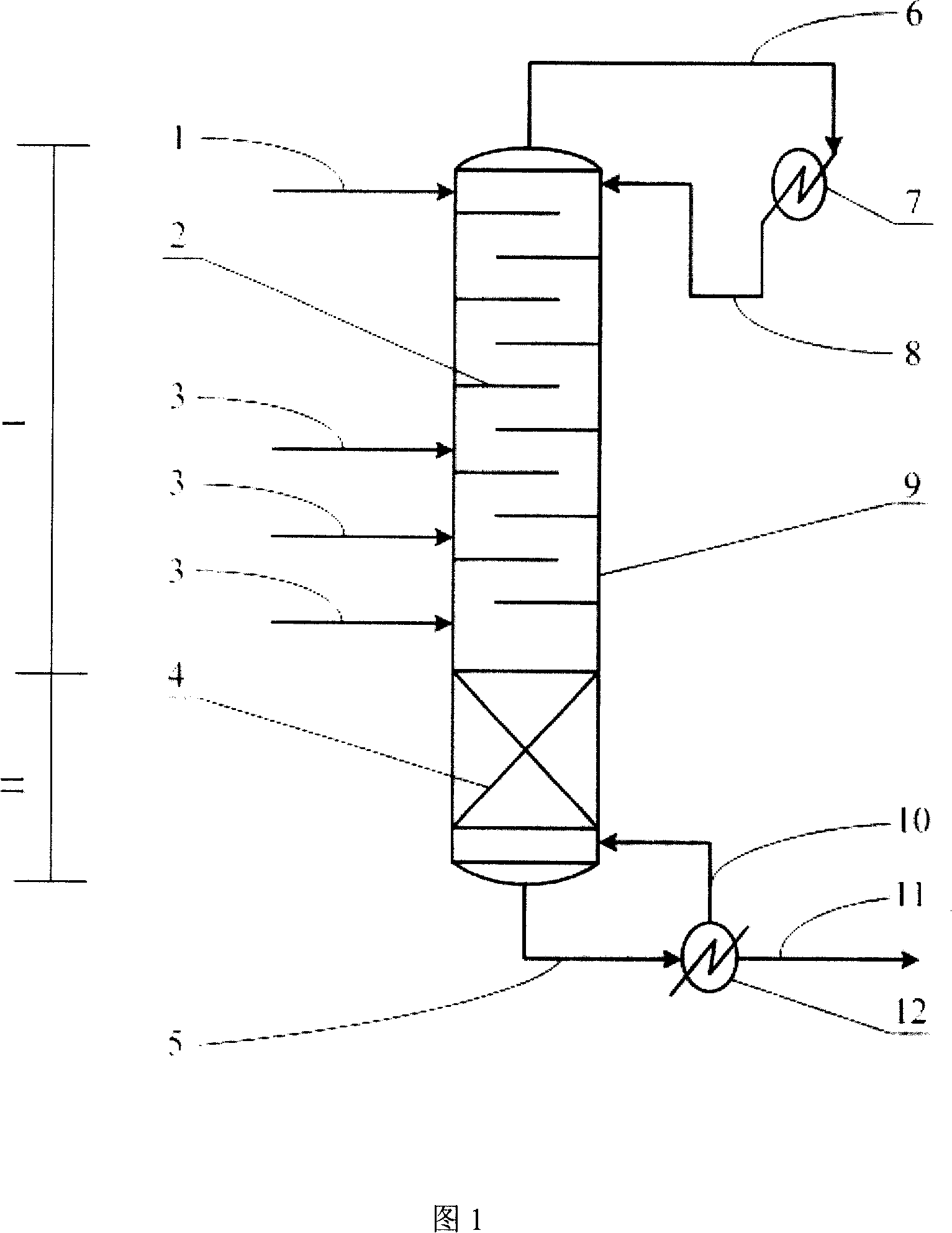
Method for producing ethylene glycol by catalysis rectification
A technology of catalytic rectification and catalytic rectification column is applied in the field of catalytic rectification to produce ethylene glycol, which can solve the problems of long process flow, large energy consumption, large equipment investment and the like, and achieve the effect of simplifying the process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation method of solid niobic acid catalyst is: take by weighing 10 grams of commercially available niobium oxalate and dissolve it in 40 milliliters of water to make niobium oxalate solution; weigh 1.0 gram of Zn(NO 3 ) 2 ·6H 2 O was dissolved in 5 ml of 10% dilute nitric acid aqueous solution to make zinc nitrate solution. Weigh α-Al 2 o 3 80 g, ZrO 2 Add 20 grams into the kneader, then add the niobium oxalate and zinc nitrate solution prepared above, and mix with α-Al 2 o 3 Fully kneaded to form a lump material, extruded into rods, dried in vacuum at 120°C for 2 hours, and calcined in air at 500°C for 4 hours to obtain solid niobic acid catalyst A. The catalyst composition is shown in Table 1.
Embodiment 2
[0028] Prepare solid niobic acid catalyst B by the method of embodiment 1, the difference is that niobium oxalate consumption is 2.4 grams, 0.18 gram Zn(NO 3 ) 2 ·6H 2 O dissolved in 20 ml water, SiO 2 / Al 2 o 3 100 grams of HZSM-5 molecular sieve carrier with a molar ratio of 140 was dried in vacuum at 110° C. for 2 hours, and calcined in air at 300° C. for 4 hours. The catalyst composition is shown in Table 1.
Embodiment 3
[0030] Prepare solid niobic acid catalyst C by the method of embodiment 1, the difference is that niobium oxalate consumption is 2.4 grams, 18 gram Zn(NO 3 ) 2 ·6H 2 O dissolved in 20 ml of water, 5 g of inorganic clay binder, 20 g of α-Al carrier 2 o 3 and SiO 2 / Al 2 o 3 80 grams of HZSM-5 molecular sieve with a molar ratio of 140, dried in vacuum at 120°C for 2 hours, and calcined in air at 700°C for 4 hours. The catalyst composition is shown in Table 1.
[0031] Table 1
[0032] Example
[0033] Other acidic or alkaline insoluble water-soluble inorganic materials and their composite materials can also improve the selectivity of ethylene glycol by adopting the catalytic rectification method of the present invention, and the specific preparation method of the catalyst provided here is not to limit the present invention range.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com