Improved technique for synthesizing dispersion blue 60
A synthesis process, a technology for disperse blue, applied in the field of improved disperse blue 60 synthesis process improvement, can solve the problems of long condensation reaction time, low reaction activity, long reaction time, etc., and achieves improved equipment utilization, reduced reaction temperature, shortened The effect of the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
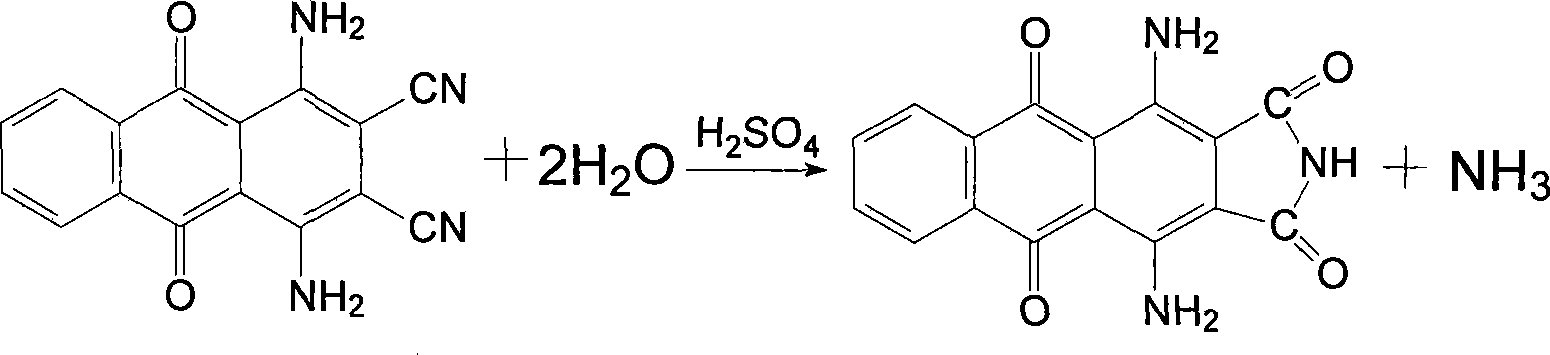
[0025] Add 40ml of concentrated sulfuric acid into a 250ml four-necked flask, slowly add 20g of 1,4-diamino-2,3-dicyanoanthraquinone after the temperature is raised, and after the addition, the temperature is raised to 70°C and the reaction is kept for two hours. The end point is detected by the pull plate. Then the temperature was lowered to 40°C, 120ml of water was slowly added dropwise to isolate, and then filtered while hot, and the filter cake was washed with hot water.
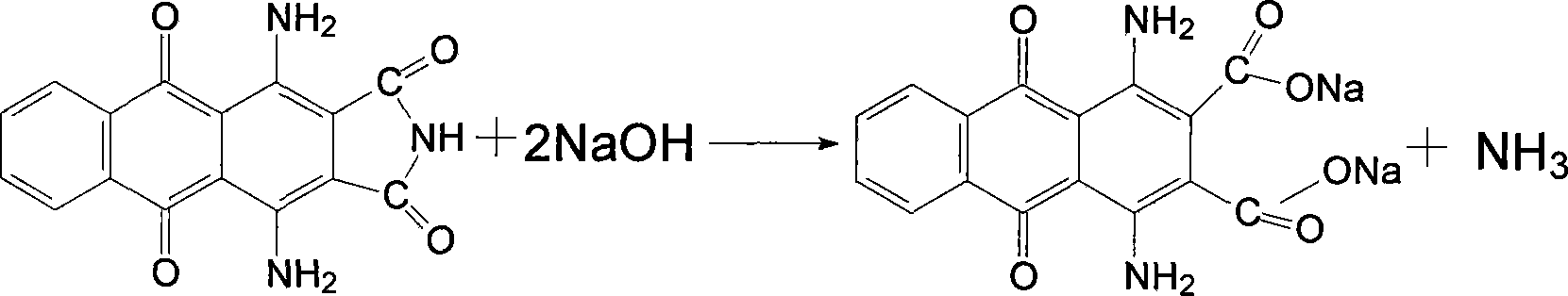
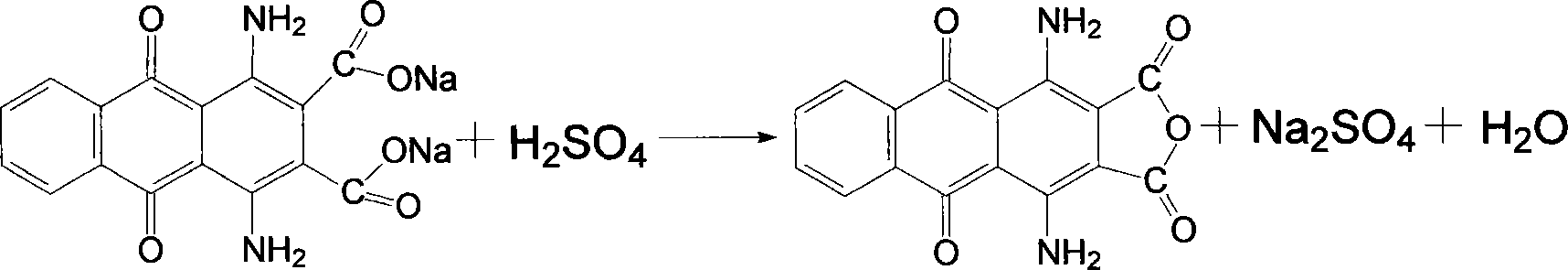
[0026] Put the above filter cake into a 250ml four-necked flask, add 120ml of water and stir evenly, then add 6g of sodium hydroxide and 2g of activated carbon, increase the temperature to 100℃ and keep the reaction for one hour, filter while hot, wash the filter residue with hot water, and combine the mother liquor And the washing liquid, in the flask, the temperature is raised to 60℃, and then 10ml of 40% sulfuric acid is slowly added dropwise. After the addition, the temperature is raised to 100℃ and the ...
Embodiment 2
[0029] Using the same feeding ratio and operation method as in Example 1, 80ml γ-ethoxypropylamine was used instead of 80ml γ-methoxypropylamine during condensation. After the same treatment, 25.1g of the product was obtained, with a total yield of 93.4%, proofing The brilliance was determined to be 0.8.
Embodiment 3
[0031] Add 40ml of concentrated sulfuric acid to a 250ml four-necked flask, slowly add 20g of 1,4-diamino-2,3-dicyanoanthraquinone after the temperature is raised, and then heat up to 70°C and keep the reaction for two hours. The pull plate detects the end point Then the temperature was lowered to 40°C, 120ml of water was slowly added dropwise to isolate, and then filtered while hot, and the filter cake was washed with hot water.
[0032] Put the above filter cake into a 250ml four-neck bottle, add 120ml of water and stir evenly, then add 6g of sodium hydroxide, heat up to 100°C and keep the reaction for one hour, filter while hot, wash the filter residue with hot water, combine the mother liquor and lotion In the flask, the temperature is raised to 60°C, and then 10ml of 40% sulfuric acid is slowly added dropwise. After the addition, the temperature is raised to 100°C and the reaction is kept for one hour, filtered while hot, the filter cake is washed with hot water and dried.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com