Process for synthesizing ethenylamidine hydrochloride by one-step method
An acetamidine hydrochloride and process technology, which is applied in the field of acetamidine hydrochloride synthesis technology, can solve the problems of long process time, unreasonable design of the concentration kettle, and reduced product yield, etc., so as to increase the methanol recovery condenser and solve the problem of mass transfer. Heat transfer issues, the effect of improving yield and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Ingredients ratio: Acetonitrile: HCL: Methanol: NH 3 =1:1.28:8.29:1.25 (molar ratio)
[0038] Raw materials: mass content of acetonitrile ≥99%, water content ≤0.05%
[0039] Methanol mass content ≥ 99.5%, moisture ≤ 0.05%
[0040] Acid methanol HCL mass content = 44-47%, moisture ≤ 0.1% CL 2 <20PPM
[0041] Ammonia Methanol NH 3 Mass content = 11-12%, moisture ≤ 0.1%
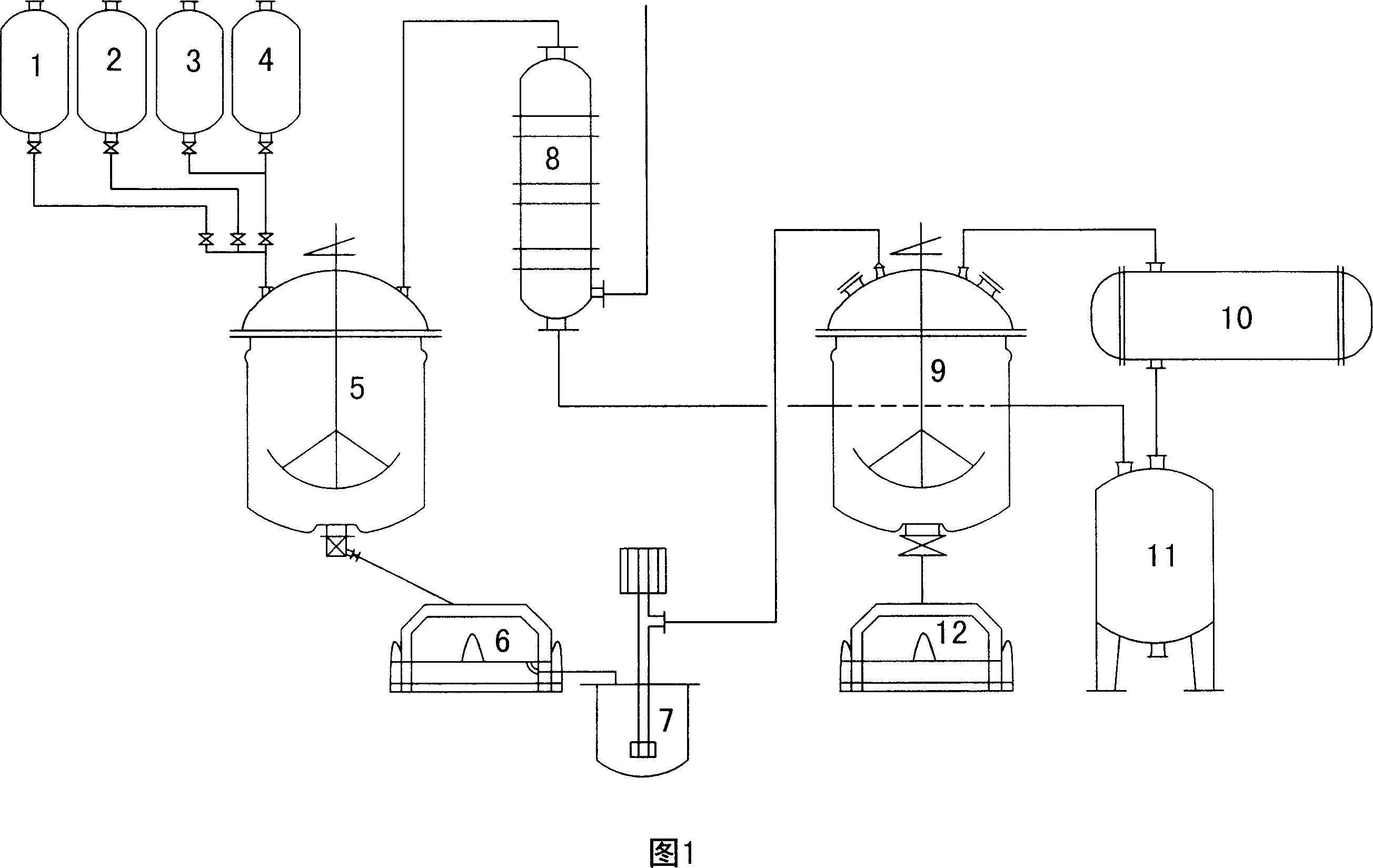
[0042] Sour methyl alcohol is put into synthetic kettle acetamidine hydrochloride synthetic kettle 5 by acid methanol high-level metering tank 2, and acetonitrile is dripped in the synthetic kettle 5 from acetonitrile high-level metering tank 1 with the speed of 60kg per hour, and initial dropwise feed temperature is at Below 0°C, add 60kg dropwise and react for 30 minutes, the temperature of the material will automatically rise above 8°C, and then add acetonitrile dropwise at 12±2°C until the drop is complete. Control the temperature at 18°C for 1 hour, keep warm at 18-22°C for 2 hours, keep warm...
Embodiment 2
[0045] Turn on the stirring of the 3000L synthesis kettle, send ice salt water, add the metered acid methanol (646kg, HCl content 45%, and the total number of HCl moles is 7.968kmol) into the synthesis kettle at one time, the material temperature drops to -5°C, and close Iced brine, start to drop about 60kg of acetonitrile and react for 30 minutes, wait for the temperature of the material to rise above 7°C, start to drop the remaining amount of acetonitrile (the total amount of acetonitrile is 275kg, totaling 6.64kmol), and use iced brine to control the temperature of the material at 14°C Below, let the feed liquid heat up slowly after dripping, and the temperature rise rate is controlled at 5-6 seconds to raise 0.1°C. When the feed temperature rises to 18°C, control the temperature at this temperature for 1 hour, and then keep it at 18-22°C for 2 Hours, keep warm at 22-26°C for 2 hours, then cool down to 10°C, add 250kg of diluted methanol to the synthesis kettle, continue to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com