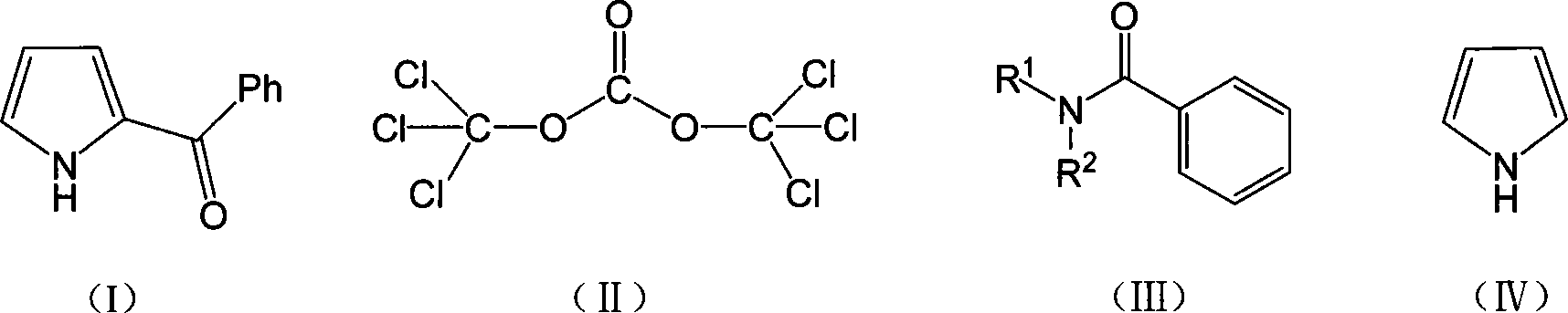
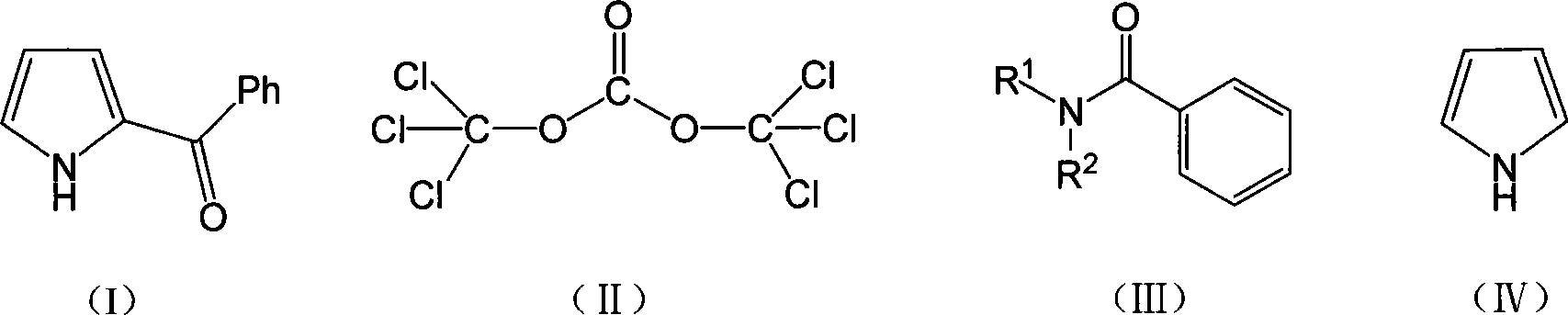
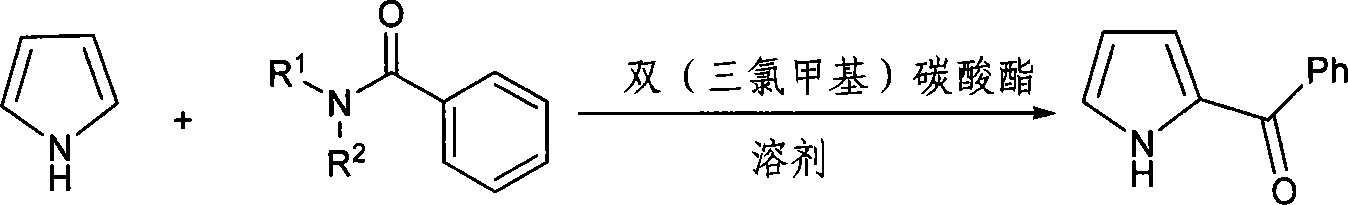
Method for synthesizing ketorolac ammonia butanetriol key intermediate compound benzoyl pyrrole
A technology of benzoylpyrrole and pyrrole, which is applied in the field of synthesis of benzoylpyrrole, a key intermediate of ketorolac tromethamine, can solve problems such as difficult treatment of phosphorus-containing wastewater, prominent environmental problems, and many reaction steps , to achieve the effects of low production cost, less waste, and good reaction performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The molar ratio of the feed material is pyrrole: bis (trichloromethyl) carbonate: N, N-disubstituted benzamide is 1: 0.33: 1, N, N-disubstituted benzamide is N, N-diethyl Benzyl benzamide and 2-methyltetrahydrofuran are used as solvent, and its consumption is 20 times of the quality of bis(trichloromethyl)carbonate.
[0022] In a 150mL four-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, add 75mmol (13.3g) of N,N-diethylbenzamide, and add 2-methyltetrahydrofuran (140.8g ) two (trichloromethyl) carbonate 25mmol (7.4g) of dissolving, solvent consumption is 20 times of two (trichloromethyl) carbonate quality, dropwise under ice bath, continue to stir 5h, intermediate is carried out insulation, Then add pyrrole 75mmol (5.03g), react for 4h, and the reaction temperature is 80°C. After the reaction is complete, add saturated brine, stir for 1 h, separate the liquids, and extract with dichloromethane. The obtained organic phase is distilled u...
Embodiment 2
[0024] The molar ratio of the feed material is pyrrole: bis(trichloromethyl) carbonate: N,N-disubstituted benzamide is 1:0.5:1.5, pyrrole is 75mmol (5.03g), N,N-disubstituted benzamide The amide is N,N-diethylbenzamide, and 2-methyltetrahydrofuran is used as a solvent, and the amount used is 20 times the mass of bis(trichloromethyl)carbonate. The reaction temperature was 0°C, the reaction time was 4 hours, and the intermediate was kept warm for 5 hours.
[0025] Other operations are the same as in Example 1, the product yield is 85.0%, and the purity is 98.2%.
Embodiment 3
[0027] The molar ratio of the feed material is pyrrole: bis(trichloromethyl) carbonate: N, N-disubstituted benzamide is 1: 0.75: 2.25, pyrrole is 75mmol (5.03g), N, N-disubstituted benzamide The amide is N,N-diethylbenzamide, and 2-methyltetrahydrofuran is used as a solvent, and the amount used is 20 times the mass of bis(trichloromethyl)carbonate. The reaction temperature is 80° C., the reaction time is 4 hours, and the intermediate is kept warm for 5 hours.
[0028] Other operations are the same as in Example 1, the product yield is 87.8%, and the purity is 96.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com