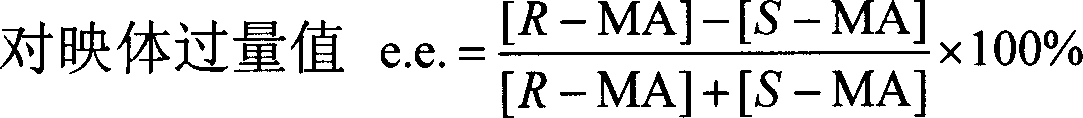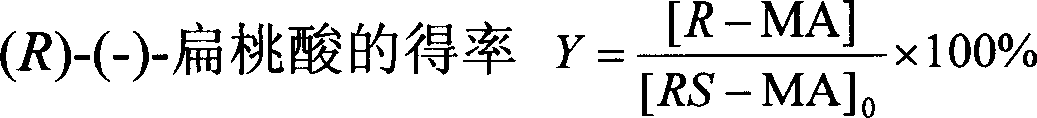Bacillus alcaligenes and method for preparing single enantiomer amygdalic acid
A technology of Alcaligenes, mandelic acid, applied in the directions of microorganism-based methods, biochemical equipment and methods, bacteria, etc., to achieve the effect of good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Microbial Culture
[0059] Inoculate 50mL fermentation medium (10g of glycerol, 15g of peptone, 8g of yeast extract, KH 2 PO 4 4g, NaCl2g, MgSO 4 0.2g, FeSO 4 ·7H 2 (00.05g, tap water 1000mL, pH7.0) pre-cultured for 15h, with the culture solution as a seed, inoculated to 200mL fermentation medium with a 10% inoculum size, and cultivated on a shaker at 160rpm at 30°C for 48h.
Embodiment 2
[0060] Example 2 Preparation of (R)-(-)-mandelic acid by resting cells
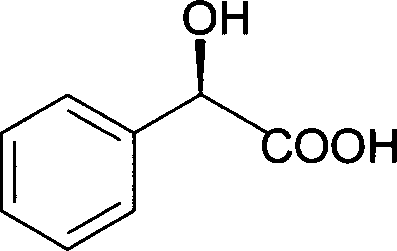
[0061] Centrifuge the harvested Alcaligenes sp.ECU0401 cells at 12,000×g for 15 minutes to obtain resting cells with a wet weight of 100 g, suspend the cells in 1000 mL of potassium phosphate buffer solution, add 3.8 g of racemic mandelic acid, and the final concentration is 25mM, shaken at 30°C and 160rpm for 14 hours, then centrifuged the reaction solution at 12,000×g for 15min to remove cells. Add 2M H to the supernatant 2 SO 4 Acidify to a pH of 1.0-2.0, add salt to saturation, extract with an equal volume of ethyl acetate, repeat three times, combine the extracts, add anhydrous sodium sulfate to dry overnight, and remove the organic solvent by rotary evaporation to obtain a crude crystal, which is passed through a silica gel column layer Analysis (benzene: ethyl acetate: formic acid, volume ratio 10:2:1) to obtain (R)-(-)-mandelic acid after purification: white powder, yield 41.5%, e.e.>99.9%, spec...
Embodiment 3
[0062] Example 3 Catalytic synthesis of (R)-(-)-mandelic acid by growing cells
[0063] In 200mL medium (peptone 15g, yeast extract 8g, KH 2 PO 44 g, NaCl2g, MgSO 4 0.2g, FeSO 4 ·7H 2 (00.05g, 1000mL of tap water, pH7.0) add 2g racemic mandelic acid, cultivate Alcaligenes sp.ECU040148 hours at 30 ℃ and constant temperature shaker of 160rpm, fermented liquid is centrifuged 15min with 12,000 * g, Remove cells. Add 2M H to the supernatant 2 SO 4 Acidify to a pH of 1.0-2.0, add salt to saturation, extract with an equal volume of ethyl acetate, repeat three times, combine the extracts, add anhydrous sodium sulfate to dry overnight, and remove the organic solvent by rotary evaporation to obtain a crude crystal, which is passed through a silica gel column layer After purification by analysis (benzene: ethyl acetate: formic acid, volume ratio 10:2:1), pure (R)-(-)-mandelic acid was obtained: white powder, yield 40.5%, e.e.>99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com