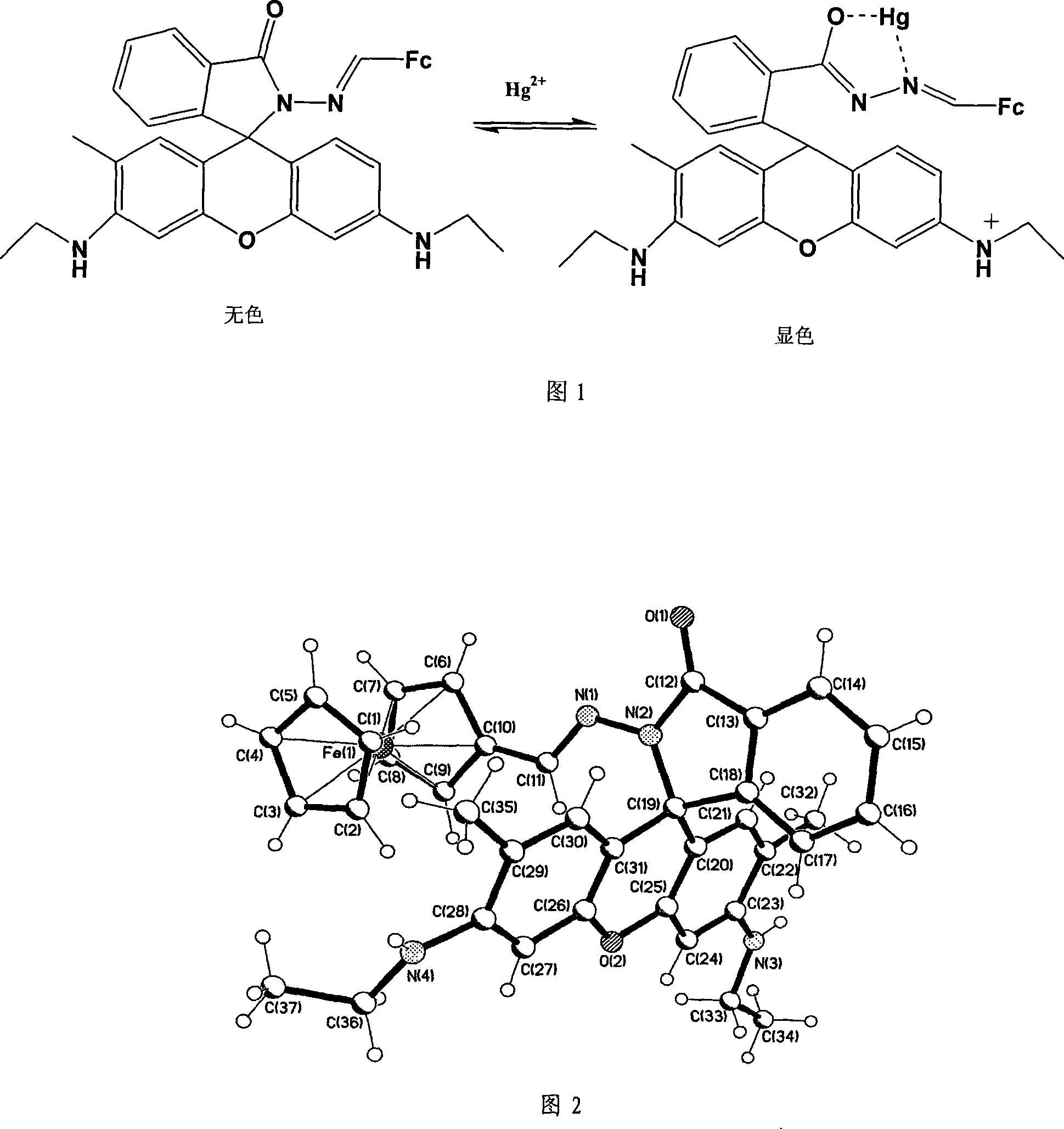
Mercury ion fluorescent color-developing agent and detecting method,measuring test paper and uses thereof
A fluorescent color reagent, mercury ion technology, applied in chemical instruments and methods, luminescent materials, chemiluminescence/bioluminescence, etc., can solve the problems of lack of water solubility of color reagents, low fluorescence yield, lack of detection metals, etc. Achieve the effect of good selective recognition, high fluorescence intensity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]Embodiment 1 (the synthesis of compound RFc)
[0030] Add 0.428 g of rhodamine-6G hydrazine compound (1 mmol), 0.214 g of ferrocene monoaldehyde (1 mmol), and 3 drops of glacial acetic acid into 30 ml of ethanol, reflux for 120 minutes, cool to room temperature, and collect the light yellow precipitate by filtration , washed with water, methanol, and anhydrous ether for several times. Vacuum dry. Yield: 0.547g, 87.5%. Elemental analysis calculated value RFc (C 37 h 36 N 4 o 2 Fe): H 5.81%, C 71.13%, N 8.97%. Analytical values: H 5.83%, C 71.22%, N 8.98%; ESI-MS: m / z 625.4, assigned to [RFc+H] + Ion peak (calculated as [RFc+H] + =625.2. 1 H NMR (DMSO-d6)H 8.041(1H, s), 7.877(1H, d, J=7.0Hz), 7.568(1H, t, J=15.0Hz), 7.521(1H, t, J=15.0Hz) , 6.978(1H, d, J=6.5Hz), 6.372(1H, s), 6.215(1H, s), 4.358(2H, s), 4.315(2H, s), 3.825(5H, s), 3.161( 4H, m, J = 182Hz), 1.851 (6H, s), 1.174 (6H, t, J = 25.5Hz); IR (solid KBr): 2360.4(s), 1705.2(s), 1620.03(m), 1619.5 (m), 146...
Embodiment 2
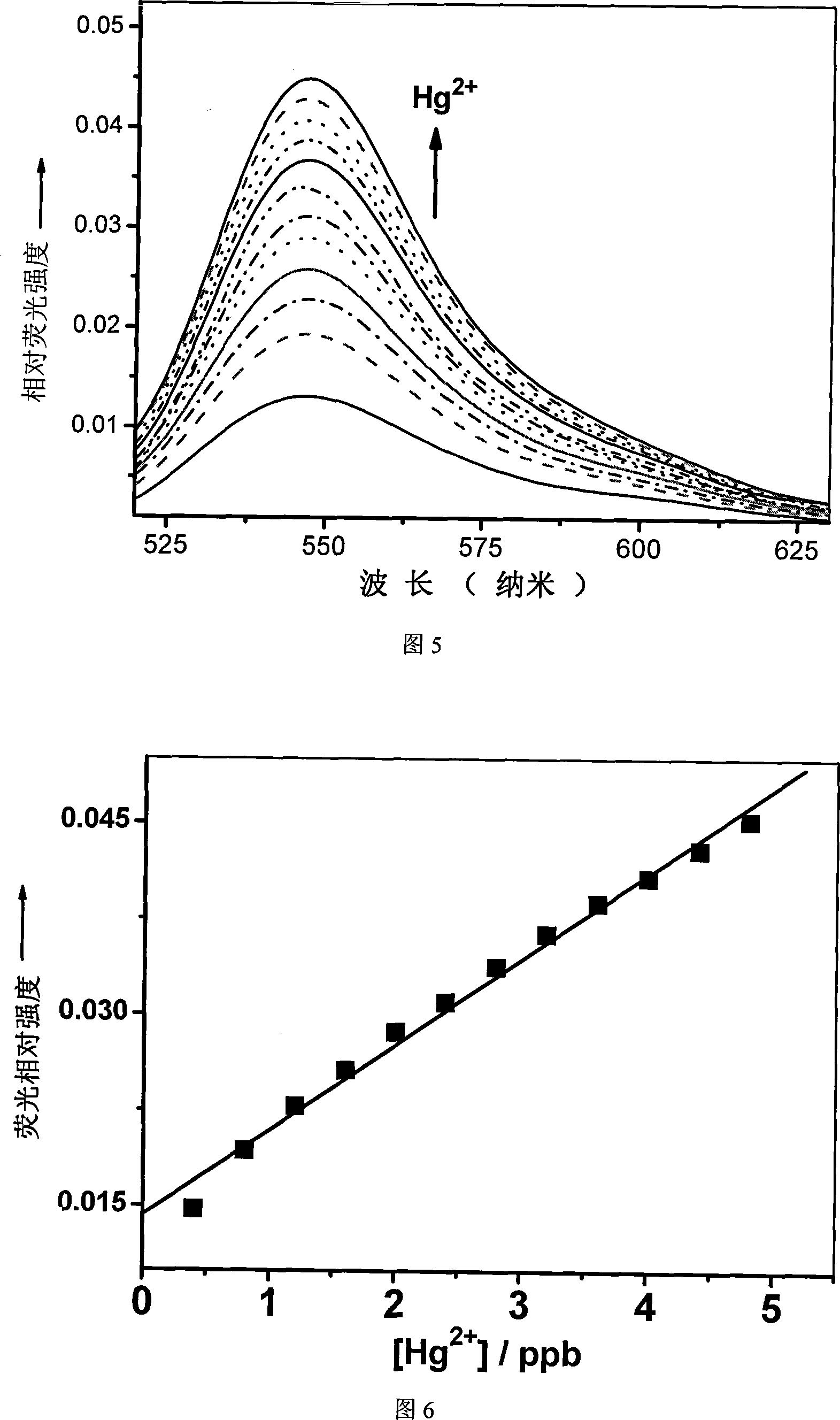
[0031] Embodiment 2 (selective experiment)
[0032] Formulate compound RFc into 1×10 -5 M aqueous solution stock solution, with metal ion salts (Li(I), Na(I), K(I), Ag(I), Mg(II), Ca(II), Cu(II), Ba(II), Perchlorates of Pb(II), Mn(II), Co(II), Ni(II), Cd(II), Zn(II), Hg(II), Fe(II) sulfates), new Prepare metal ion stock solution (concentration 10 -2 M) for experiments. In the test experiment, take compound RFc stock solution 2mL, and take other metal ion solutions so that Na in the final solution + , K + , Ca 2+ and Mg 2+ The ion is 125 times that of compound RFc, Hg 2+ Ions are 25 times that of compound RFc, and other metal ions are 50 times that of compound RFc, adjust the concentration for testing, excite at 500nm, and measure the fluorescence spectrum. The results are shown in Figure 3.
Embodiment 3
[0033] Embodiment 3 (high concentration fluorescence titration working curve)
[0034] Take by weighing the compound RFc0.0624 gram of embodiment 1, be dissolved in the N, N-dimethylformamide (DMF) of 100mL, obtain 10 -3 M's solution. Use a pipette to pipette 1 mL of the above solution, dilute to 100 mL with double distilled water in a volumetric flask, and prepare 1×10 -5 M stock solution (L1). Weigh 0.0203 g of perchloric acid perchlorate hexahydrate, dissolve in water, and prepare 4.0×10 with a 10ML volumetric flask -3 M standard stock solution (F1). Measure 12.0 ml of RFc stock solution L, add the calculated amount of mercury ion stock solution F1, prepare a standard test solution, excite at 500nm, and test its fluorescence intensity at 550nm. The test results are shown in Figure 4. Calculate the compound RFc of embodiment 1 and the binding constant of mercury ion with nonlinear least square method to be K=2.15×10 4 m -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com