Highly effective production method for recombinant alpha-glucanase fusion protein and related expression carrier and bacterial strain
A technology of glucanase and fusion protein, which is applied in the field of genetic engineering, can solve the problems of complex purification steps of α-glucanase, and achieve the effects of reducing costs, simplifying the purification process, and increasing the expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
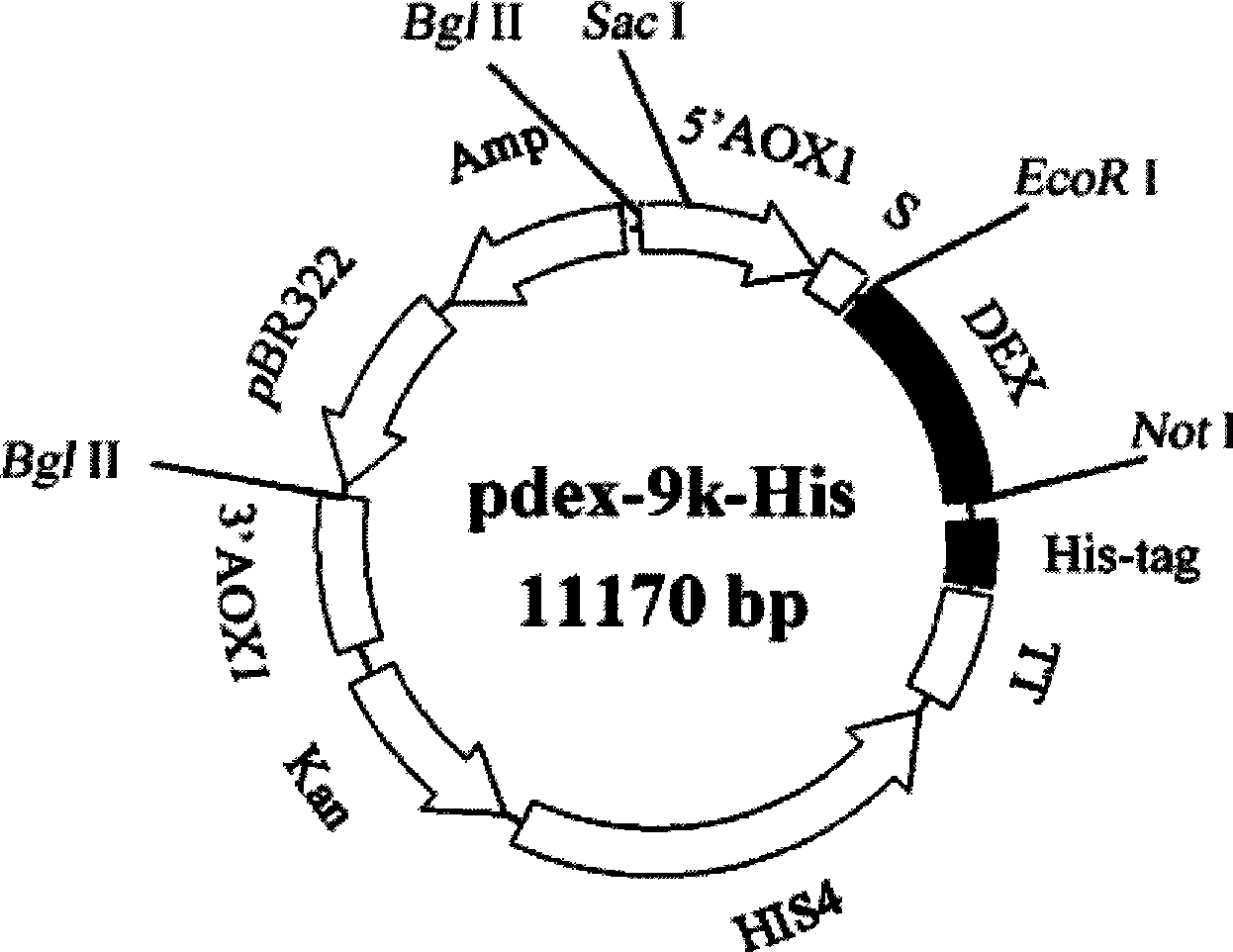
[0046] Example 1 Construction of recombinant Pichia pastoris expression vector pdex-9k-His
[0047] (1) Construction of pPIC9k-His expression vector
[0048] A pair of primers were synthesized according to the multiple cloning restriction site and the restriction site at His4 of Pichia expression vector pPIC9k (Invitrogen), the sequence of which was as follows: upstream primer (with 6 His-tag gene sequences (SEQ ID NO :3)), 5'-ATC GCGGCCGC G CATCATCATCATCATCATCAT TAATAGAATTAATTCG-3' (the underlined part is the Not I restriction sequence, and the wavy part is the His-tag gene sequence); downstream primer, 5'-CGG GTC GAC AATGTTCGTCAAAAATG-3' (the single underlined part is the SalI restriction sequence); using the pPIC9k (Invitrogen) plasmid as a template, a 1979bp DNA fragment was amplified by the following PCR reaction: PCR reaction was performed with a total volume of 50 μl, denatured at 94°C for 5 minutes, Denaturation at 94°C for 30s, annealing at 62°C for 30s, extensi...
Embodiment 2
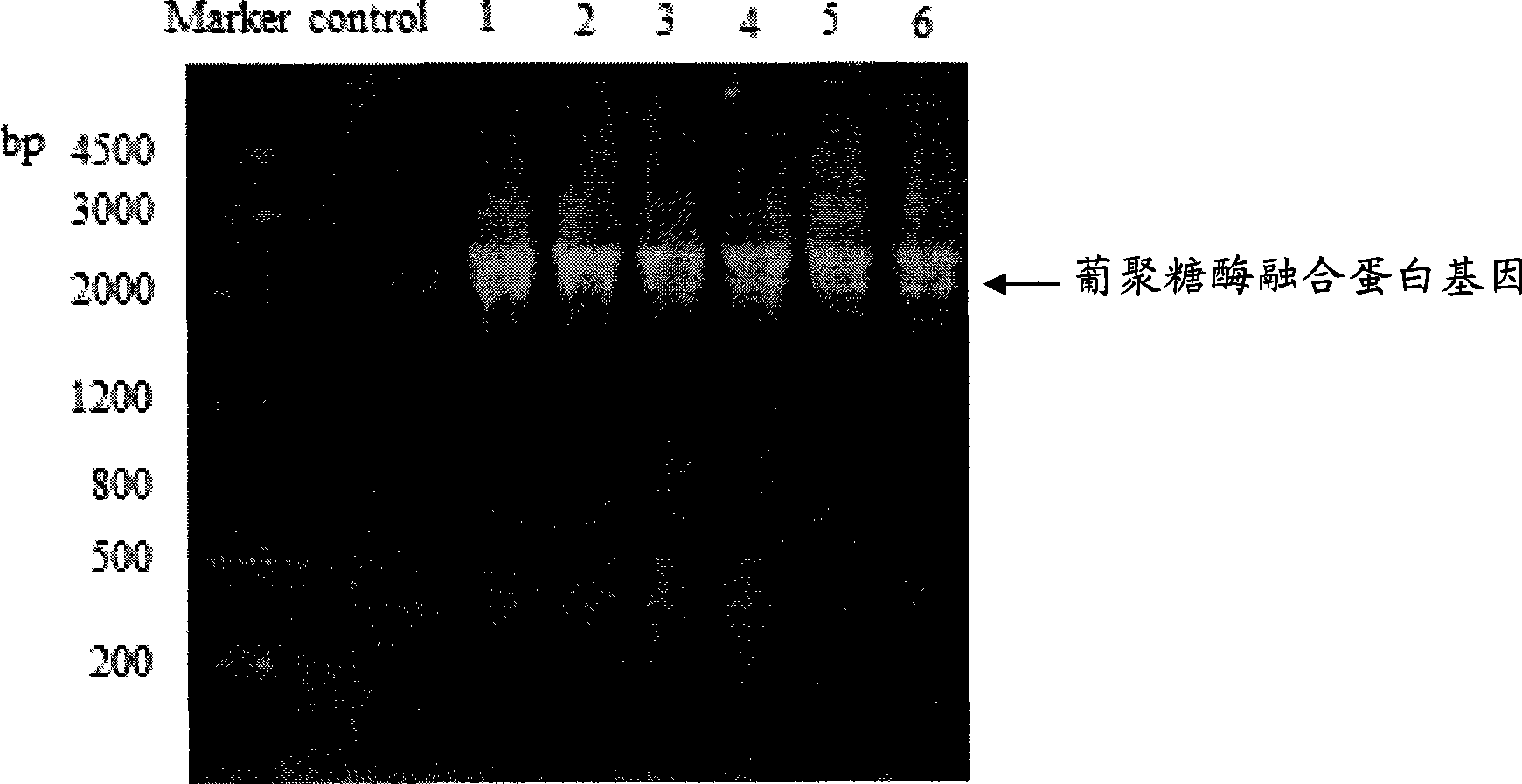
[0051] Example 2 Obtaining of highly expressed recombinant Pichia pastoris strain
[0052] (1) Transformation of recombinant Pichia pastoris expression vector pdex-9k-His and screening of positive clones
[0053] Recombinant Pichia pastoris expression vector pdex-9k-His was linearized by complete digestion with Sac I, then transformed into Pichia pastoris GS115 strain (Invitrogen) by electric shock method, and the transformation was screened on the basic medium MGY plate without histidine. homologous recombination His + clone.
[0054] Using YPD plates containing different concentrations of G418, the copy number of the exogenous gene integrated into the genome can be estimated. -5 %, glycerol 1.5%, agarose 1.5%) the transformants on the plate were eluted and suspended, after slight shaking, diluted appropriate times to 10 5 Cell concentrations of 1.0, 2.0, 3.0, and 4.0 mg / ml of G418 were spread on YPD plates respectively, and single colonies of transformants that could grow...
Embodiment 3
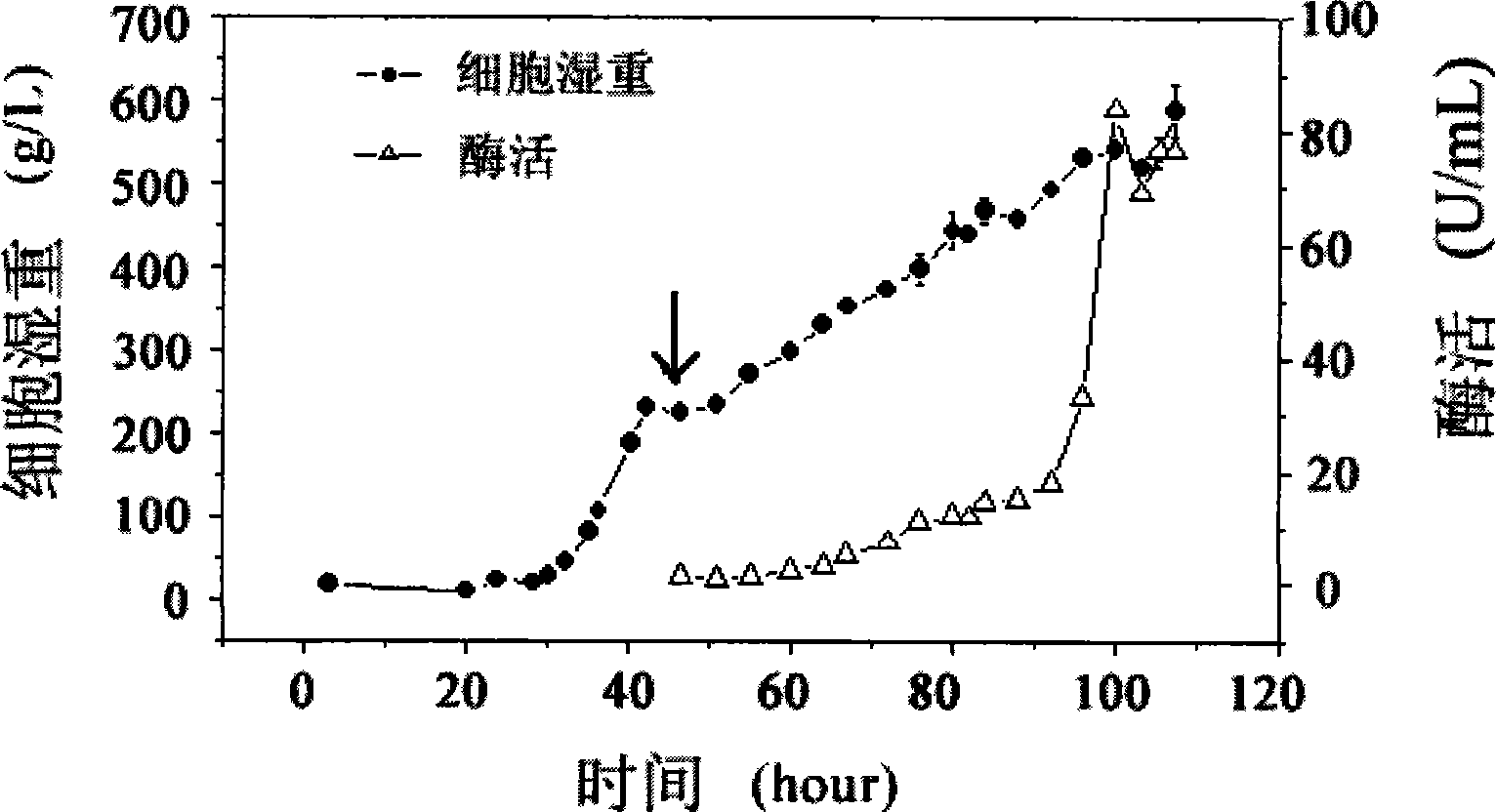
[0057] Example 3 High-density fermentation-induced expression of recombinant α-glucanase fusion protein
[0058] Add 3L of fermentation base medium to a 5L fermenter, sterilize at 121°C for 30 minutes, adjust the temperature to 28°C, adjust the pH to 6.0 with ammonia water, add PTM4.5ml / L, insert the seeds with 10% inoculum, and ferment Dissolved oxygen was maintained at 30%.
[0059] see image 3 , the fermentation is divided into two stages: the first stage is the cultivation stage, which is the growth period of the bacteria, that is, after the seed is inserted, it is cultivated for about 37 hours. At this time, the bacteria have basically exhausted the glycerin in the fermenter. The pH drops and the dissolved oxygen suddenly rises. At this point, 50% glycerin (containing PTM, 4.5ml / L) was added, and the feeding speed was 18ml L -1 h -1 , and continued for 6 hours until the wet weight of the bacteria reached 232g / L, that is, entered the second stage. The second stage is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com