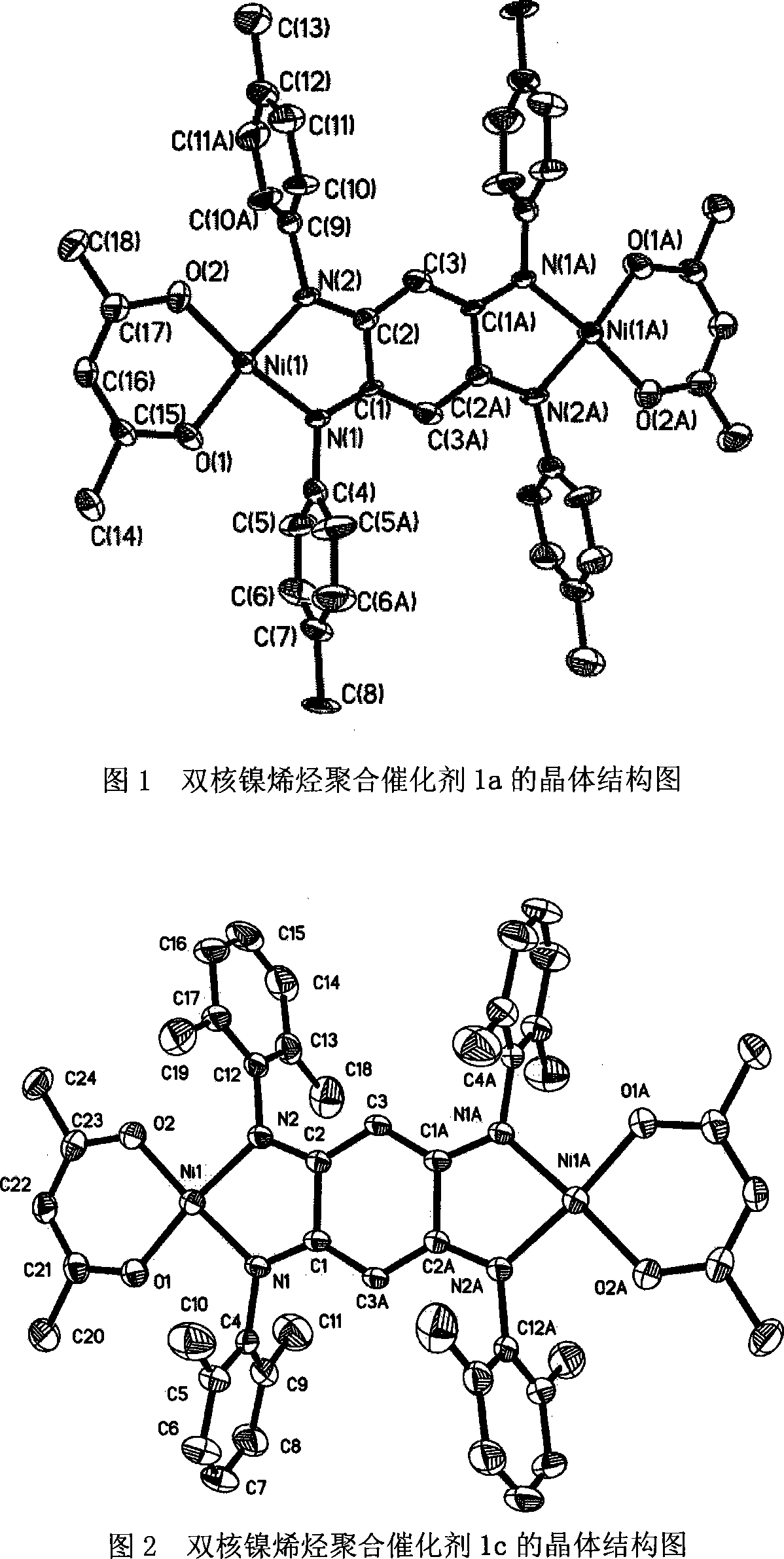
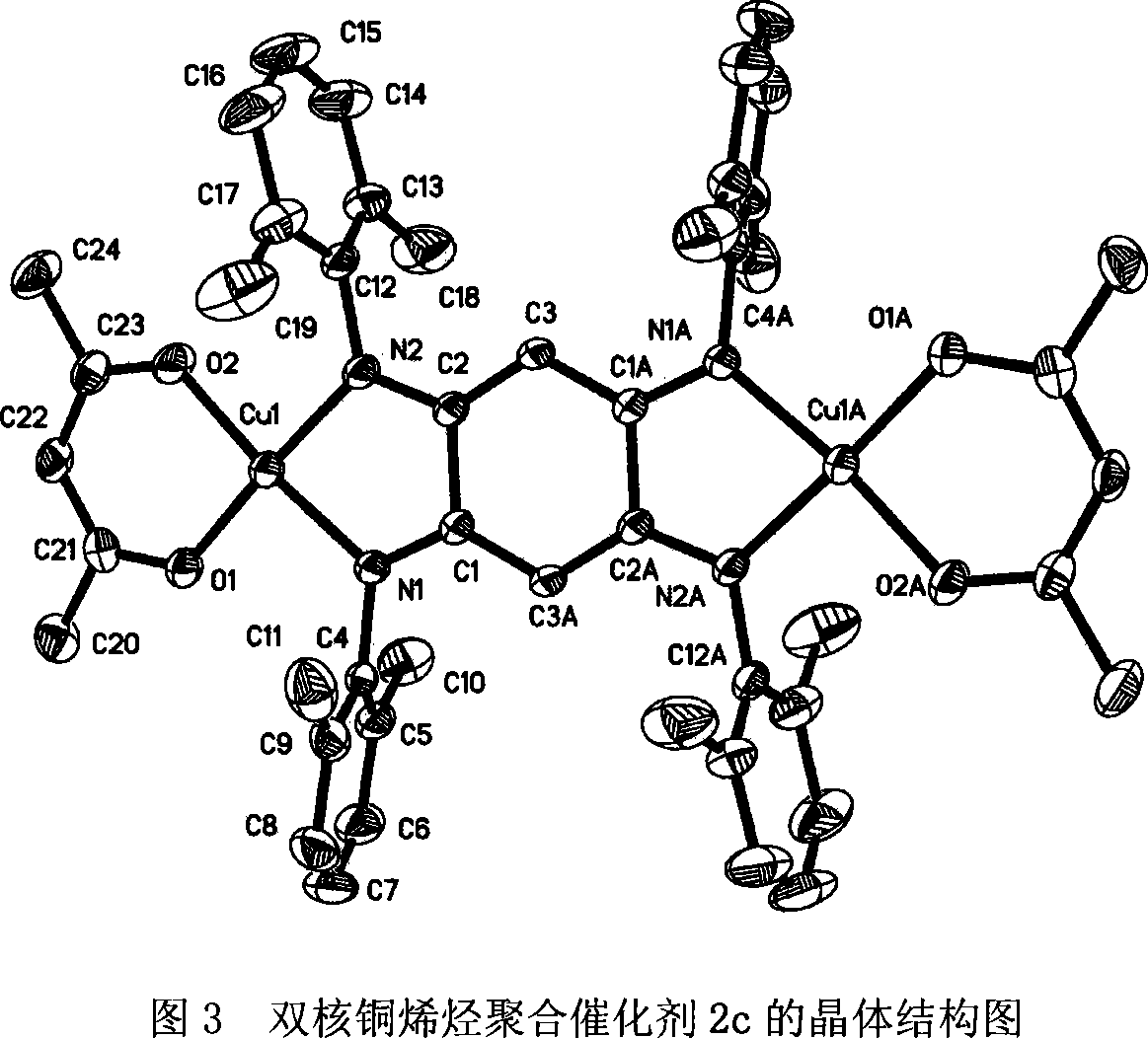
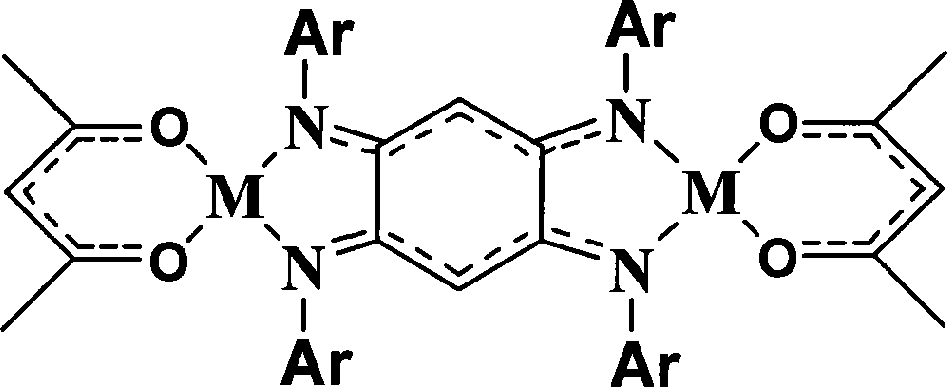
Double-core nickel, copper olefin polymerization catalyst, preparation method and application thereof
A technology of polymerization catalyst and copper olefin, which is applied in the field of copper olefin polymerization catalyst and its preparation, binuclear nickel, can solve the problems of low molecular weight of polyolefin, narrow molecular weight distribution of polyolefin, poor processability, etc., and achieve the effect of high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]Example 1: Preparation of Ligand L1:
[0029] Under nitrogen protection, 1,3-bis(2,6-diisopropylphenyl)imidazolium salt (0.1 mmol), NaOt-Bu (0.1 mmol), Pd(OAc) 2 (0.05mmol), toluene (60mL) was placed in a 250ml Schlenk bottle, stirred at 80°C for 10 minutes, then added 1,2,4,5-tetrabromobenzene (5.00mmol), p-toluidine (35mmol), NaOt- Bu (20mmol), reacted at 110°C for about 14h, cooled to room temperature, added n-hexane 60ml, filtered, CH 2 Cl 2 After washing to remove inorganic salts, the mixture was stirred at room temperature for 2 days under an oxygen atmosphere, and the solvent was removed to obtain 1.49 g of brick-red solid powder with a yield of 60%.
Embodiment 2
[0030] Example 2: Preparation of Ligand L2:
[0031] Under nitrogen protection, 1,3-bis(2,6-diisopropylphenyl)imidazolium salt (0.15mmol), NaOt-Bu (0.25mmol), Pd(OAc)2 (0.05mmol), 1, 4-Dioxane (60mL) was placed in a 250ml Schlenk bottle, stirred at 80°C for 10 minutes, and then added 1,2,4,5-tetrabromobenzene (5.00mmol), o-methylaniline (50mmol), NaO t -Bu (20mmol), reacted at 110°C for about 14h, cooled to room temperature, added n-hexane 60ml, filtered, CH 2 Cl 2 Washed to remove inorganic salts, placed in an oxygen atmosphere and stirred at room temperature for 2 days to remove CH 2 Cl 2 , to obtain 1.38 g of red solid powder with a yield of 57%.
Embodiment 3
[0032] Example 3: Preparation of Ligand L3:
[0033] Under nitrogen protection, 1,3-bis(2,6-diisopropylphenyl)imidazolium salt (0.1 mmol), NaOt-Bu (0.1 mmol), PdCl 2 (0.05mmol), xylene (60mL) was placed in a 250ml Schenk bottle, stirred at 80°C for 10 minutes, and then added 1,2,4,5-tetrabromobenzene (5.00mmol), 2,6-dimethylaniline ( 20 mmol), NaO t-Bu (50 mmol), reacted at 110 ° C for about 14 h, cooled to room temperature, added n-hexane 60 ml, filtered, CH 2 Cl 2 Washed to remove inorganic salts, placed in an oxygen atmosphere and stirred at room temperature for 2 days to remove CH 2 Cl 2 , 0.95 g of orange-red solid powder was obtained, and the yield was 35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com