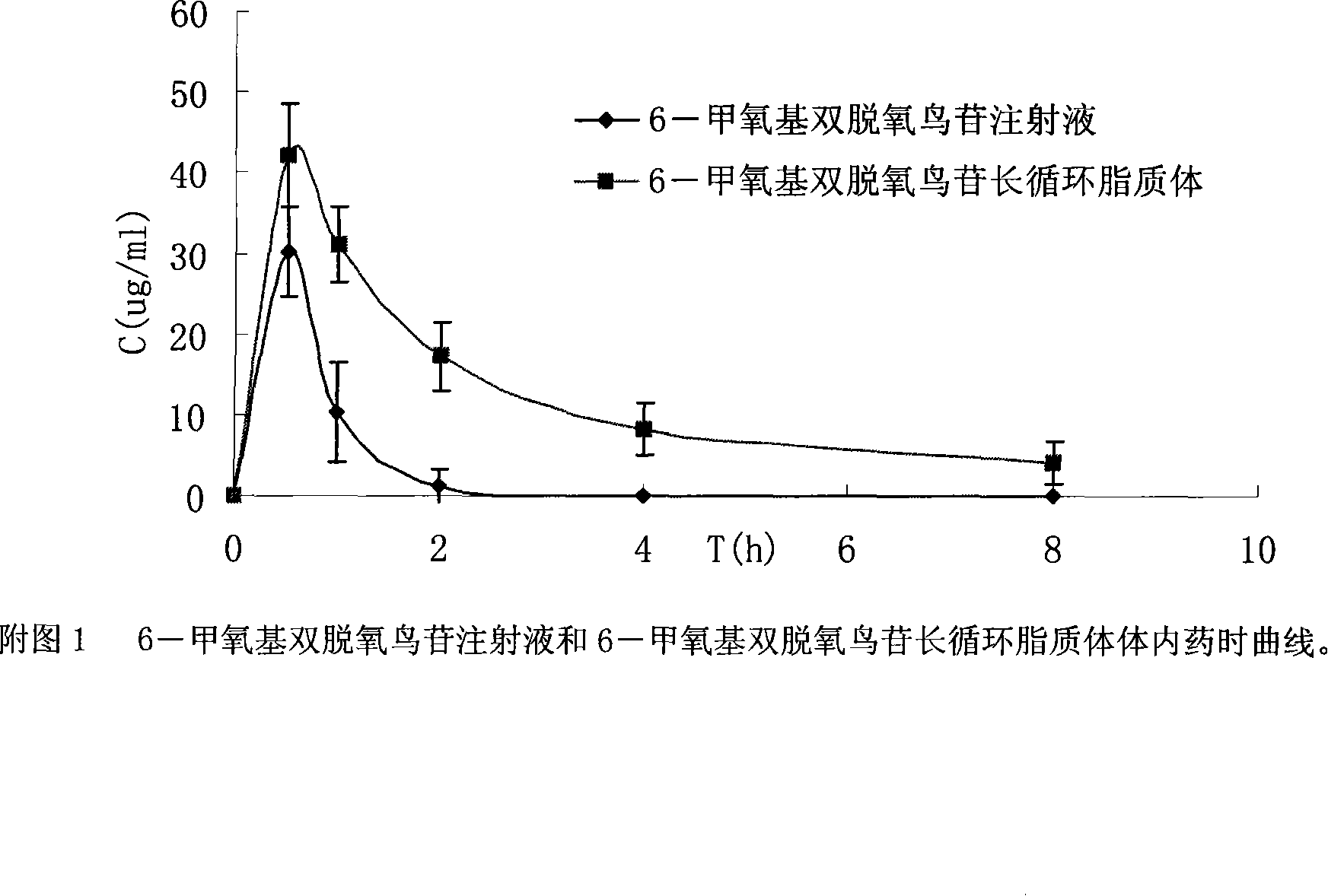
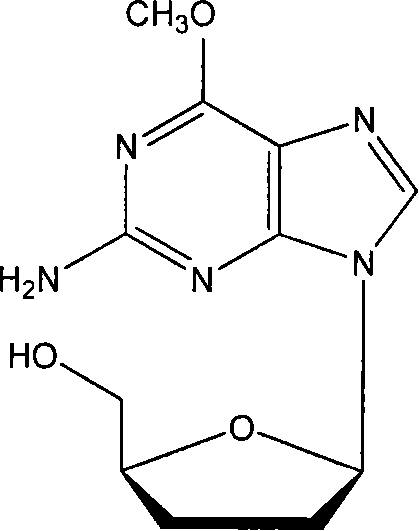
6-methocy bideoxy bideoxy guanosine long circulating liposome preparation and preparing method
A liposome preparation and liposome technology, which are applied in the field of 6-methoxydideoxyguanosine long-circulating liposome preparation and its preparation, can solve the problem of short biological half-life, impact on patients' life and work, and occupation of hospital resources. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 6-Methoxydideoxyguanosine 0.051g
[0036] Hydrogenated soybean phosphatidylcholine 0.4220g
[0037] PEG5000-PG0.3645g
[0038] Cholesterol 0.1760g
[0039] After dissolving hydrogenated soybean phosphatidylcholine and cholesterol in 50ml of absolute ethanol respectively, the ethanol was removed at 50°C with a rotary evaporator, and 15ml of 6-methoxydideoxyguanosine solution (3.3mg / ml) was added to make It is fully infiltrated and stirred with a vortex mixer to prepare a liposome suspension. Add 1.21ml of a distilled aqueous solution of polyethylene glycol 5000-phosphatidylglycerol (PEG5000-PG) (equivalent to 0.475mol% of the total lipid mass of the mixed lipids) to the above-mentioned liposome suspension, heat at 60°C For 30 minutes, modify the liposome surface with PEG5000-PG. The final liposome suspension was prepared by sequentially passing through filters (0.2um filter 2 times, 0.1um filter 2 times) in a high pressure homogenizer (Niro Soavi) at 60°C.
Embodiment 2
[0041] 6-Methoxydideoxyguanosine 0.060g
[0042] Hydrogenated soybean phosphatidylcholine 0.4120g
[0043] PE63000-PE 0.3845g
[0044] Cholesterol 0.1520g
[0045] After dissolving hydrogenated soybean phosphatidylcholine and cholesterol in 25ml of ethanol at room temperature, the ethanol was removed at 50°C using a rotary evaporator, and 10ml of 6-methoxydideoxyguanosine solution (6.0mg / ml) was added to make it Fully infiltrate and stir with a vortex mixer to prepare a liposome suspension. Add a distilled aqueous solution containing polyethylene glycol 3000-phosphatidylcholine (PEG3000-PE) (equivalent to 0.475mol% of the total lipid mass of the mixed lipids) to the above-mentioned liposome suspension, and successively The final liposome suspension was prepared by passing through filters (0.2 um filter 2 times, 0.1 um filter 3 times) in a high pressure homogenizer (Niro Soavi).
Embodiment 3
[0047] 6-Methoxydideoxyguanosine 0.060g
[0048] Lecithin 0.4520g
[0049] PEG4000-PE 0.1845g
[0050] Cholesterol 0.2020g
[0051] After dissolving lecithin and cholesterol in 25ml of ethanol heated at room temperature, use a rotary evaporator to remove ethanol at 55°C, add 10ml of 6-methoxydideoxyguanosine solution (6.0mg / ml) to make it fully infiltrated, Prepare a liposome suspension by stirring with a vortex mixer. Add a distilled aqueous solution of polyethylene glycol 4000-phosphatidylethanolamine (PEG4000-PE) (equivalent to 0.475mol% of the total lipid mass of the mixed lipids) to the above liposome suspension, and pass through high pressure successively at 65°C Filters (0.2um filter 2 times, 0.1um filter 2 times) in a machine homogenizer (Niro Soavi) were used to prepare the final liposome suspension.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com