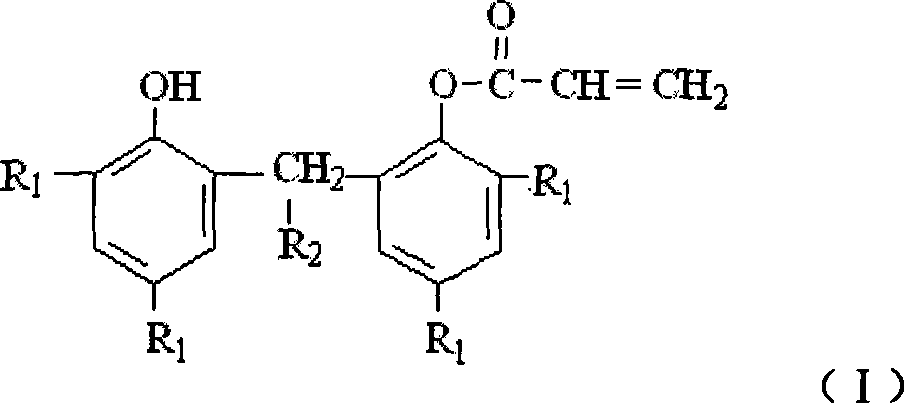
Method for preparing bisphenolmonoacryates compounds antioxidant
A monoacrylate and compound technology, which is applied in the field of preparation of bisphenol monoacrylate compound antioxidants, can solve the problems of increased equipment investment and processing costs, waste of triethylamine raw materials, troublesome processing, etc., and achieves reduction in processing process, facilitate industrial production, and improve the effect of molar yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A one-step synthesis process is adopted. Add 2,2'-ethylene bis(4,6-di 1691g (3.424mol) of p-amyl)phenol, 259g (3.596mol) of acrylic acid, and 7800mL of n-hexane. The air in the kettle was replaced with nitrogen. Heat, stir, and raise the temperature to 40-50°C. Keep the temperature constant, and start to add 194g (1.259mol) of phosphorus oxychloride raw material dropwise, and finish adding in 40-50 minutes. While adding phosphorus oxychloride dropwise, 445 g (4.405 mol) of triethylamine raw material was added dropwise, and the time for dropping the triethylamine raw material was 120 to 130 minutes. After adding the triethylamine raw material, the temperature of the reaction solution was raised to 63-66°C. At 63-66°C, keep warm for 150-160 minutes.
[0033] After the reaction is finished, the temperature of the reaction solution is cooled to room temperature, and the material is discharged, and the obtained reaction mixture is a solid-liquid mixture. After filtering,...
Embodiment 2
[0036] Add 2,2'-ethylene bis(4,6-di 1691g (3.424mol) of p-amyl)phenol, 246g (3.424mol) of acrylic acid, and 7800mL of n-hexane. The air in the kettle was replaced with nitrogen. Heat, stir, and raise the temperature to 40-50°C. Keep the temperature constant, and start to add 176g (1.141mol) of phosphorus oxychloride raw material dropwise, and finish adding in 50-60 minutes. While adding phosphorus oxychloride dropwise, 346g (3.424mol) triethylamine raw material was added dropwise, and the synthesis reaction process was completed in the same way as in Example 1. As a result, 1436 g of 2-[1-(2-hydroxyl-3,5-dipentylphenyl)-ethyl]-4,6-dipentylphenyl acrylate of the present invention was obtained. Appearance is white or light yellow crystal, melting point: 117.9-118.4. , HPLC purity: 99.4%. The product molar yield is 76.1%. Elemental analysis: C, 81.2; H, 9.9; O, 8.8. (Theoretical: C, 81.0; H, 10.2; O, 8.8).
Embodiment 3
[0038] Add 2,2'-ethylene bis(4,6-di 1691g (3.424mol) of p-amyl)phenol, 295g (4.109mol) of acrylic acid, and 7800mL of n-hexane. The air in the kettle was replaced with nitrogen. Heat, stir, and raise the temperature to 40-50°C. Keep this temperature constant, start to drop 254g (1.644mol) of phosphorus oxychloride raw material, and add it in 50-60 minutes. While adding dropwise phosphorus oxychloride, dropwise add 664g (6.574mol) triethylamine raw material, complete the synthetic reaction process by the same method of embodiment 1. As a result, 1412 g of 2-[1-(2-hydroxy-3,5-dipentylphenyl)-ethyl]-4,6-dipentylphenyl acrylate of the present invention was obtained. Appearance is white or yellowish crystal, melting point: 117.3-118.5. , HPLC purity: 99.0%. The product molar yield is 74.5%. Elemental analysis: C, 81.1; H, 9.8; O, 9.1. (Theoretical: C, 81.0; H, 10.2; O, 8.8).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com