Liquefaction catalytic conversion method for producing dimethyl ether with methanol
A fluidized catalysis and dimethyl ether technology, which is applied in the dehydration of hydroxyl-containing compounds to prepare ether, the formation/introduction of ether groups/acetal groups/ketal groups, and the preparation of ethers, etc. The problems of short service life of the catalyst, a large amount of residual acid and waste water, etc., achieve the effect of being beneficial to methanol conversion, increasing the processing capacity, gas-solid mass transfer and high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
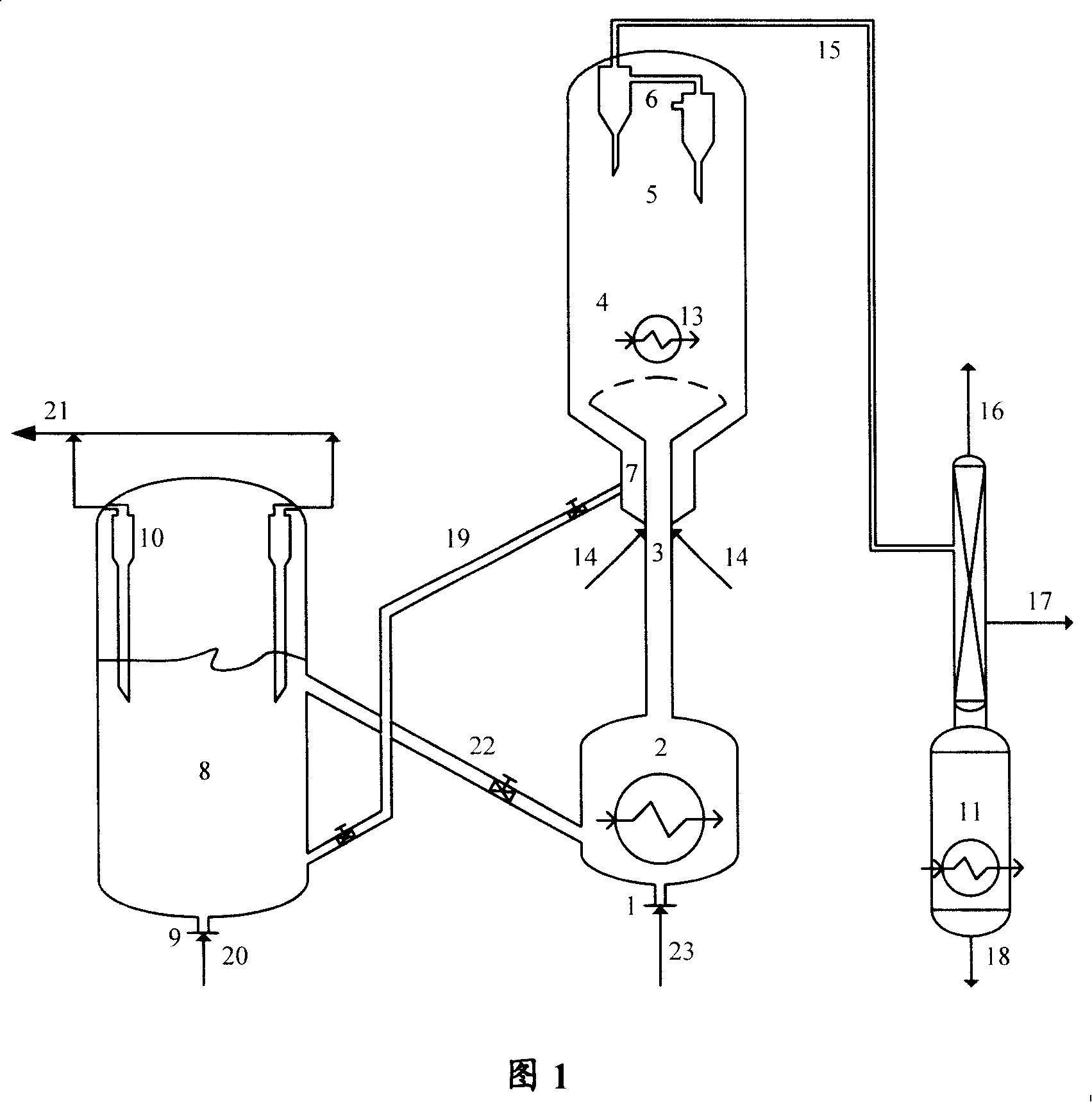
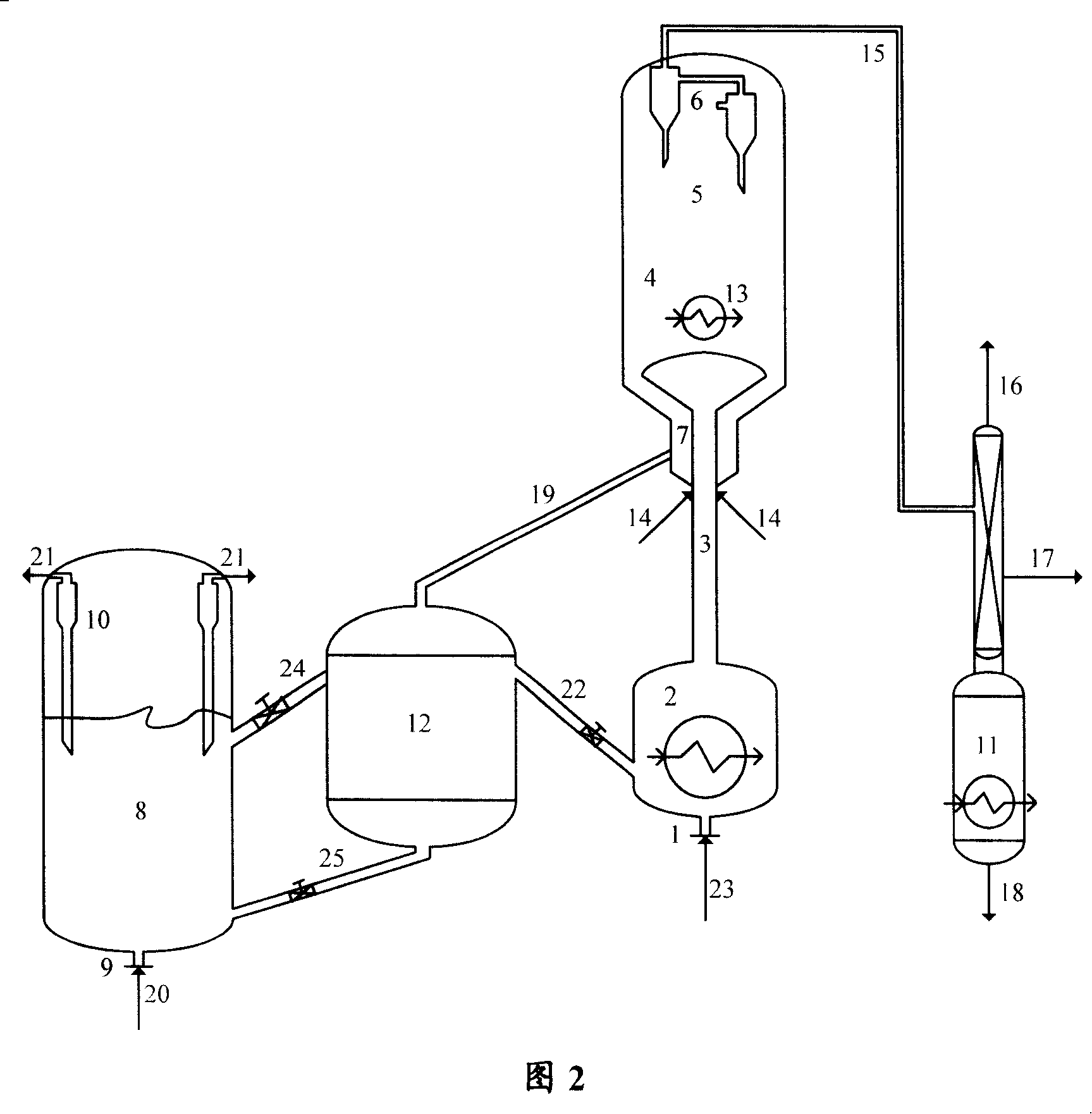
[0049] Methanol raw material, regenerated catalyst A cooled to 400°C and water vapor enter the circulating fluidized bed reactor, under pressure of 0.11MPa (gauge pressure), temperature of 400°C, liquid hourly space velocity for 4 hours -1 , react under the condition of agent-alcohol ratio 2, remove heat from the circulating fluidized bed reactor during the reaction, and the weight of the low-temperature catalyst taken from the reactor to the heat extraction section of the regenerant accounts for 15% of the total weight of the catalyst in the reactor , separating the reactant flow and the raw catalyst, wherein the reactant flow is separated to obtain the target product dimethyl ether, and the raw catalyst is stripped and regenerated in turn, and then enters the regenerated catalyst to take the hot section to cool and return to the reactor for recycling.
[0050] The operating conditions and product distribution are listed in Table 2. As can be seen from Table 2, the conversion...
Embodiment 2
[0055] Methanol raw material, regenerated catalyst A cooled to 350°C and water vapor enter the circulating fluidized bed reactor, under pressure of 0.11MPa (gauge pressure), temperature of 350°C, liquid hourly space velocity of 2.5 hours -1 1. Reaction under the condition of agent-alcohol ratio of 10. During the reaction, the heat is removed from the circulating fluidized bed reactor, and the low-temperature catalyst is not taken from the reactor to the heat extraction section of the regenerant, that is, there is no catalyst circulation in the reactor, and the separation reaction The stream and the raw catalyst, wherein the reactant stream is separated to obtain the target product dimethyl ether, the raw catalyst is stripped in turn and mixed with the high-temperature regenerated catalyst, and 40% by weight of the mixed catalyst enters the regenerator and burns, and the other part is After entering the regenerated catalyst heat extraction section, it is cooled and returned to t...
Embodiment 3
[0058] Methanol raw material, regenerated catalyst A cooled to 200°C and water vapor enter the circulating fluidized bed reactor, under pressure of 0.11MPa (gauge pressure), temperature of 200°C, liquid hourly space velocity of 0.5 hours -1 1. Reaction under the condition of agent-alcohol ratio of 18. During the reaction process, the heat is removed from the circulating fluidized bed reactor, and the low-temperature catalyst is not taken from the reactor to the heat extraction section of the regenerant, that is, there is no catalyst circulation in the reactor, and the separation reaction The stream and the raw catalyst, wherein the reactant stream is separated to obtain the target product dimethyl ether, and the raw catalyst is stripped and regenerated in turn, and then enters the regenerated catalyst to take the heat section to cool and return to the reactor for recycling.
[0059] The operating conditions and product distribution are listed in Table 3. As can be seen from Ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com