Duloxetine hydrochloride sustained release medicine
A technology for duloxetine hydrochloride and sustained-release drugs, which is applied in the field of duloxetine hydrochloride sustained-release drugs, can solve problems such as adverse reactions and limit clinical drug doses, achieve strong drug efficacy, enhance drug compliance and clinical treatment Effect, the effect that the manufacturing process is easy to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
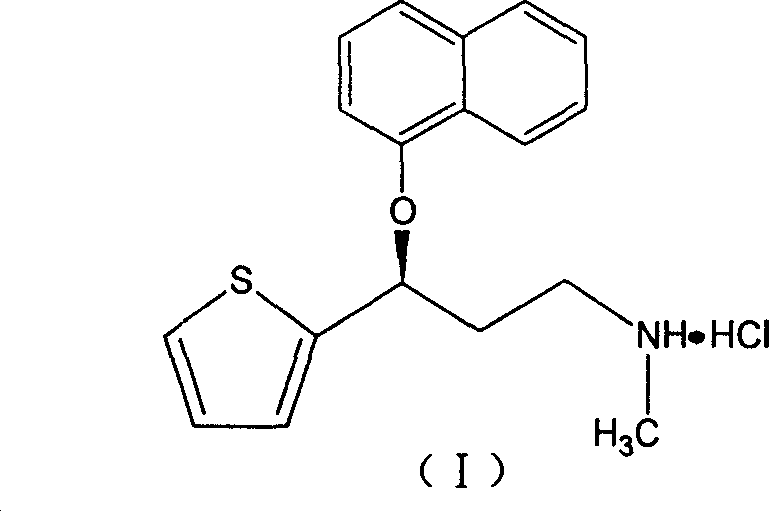
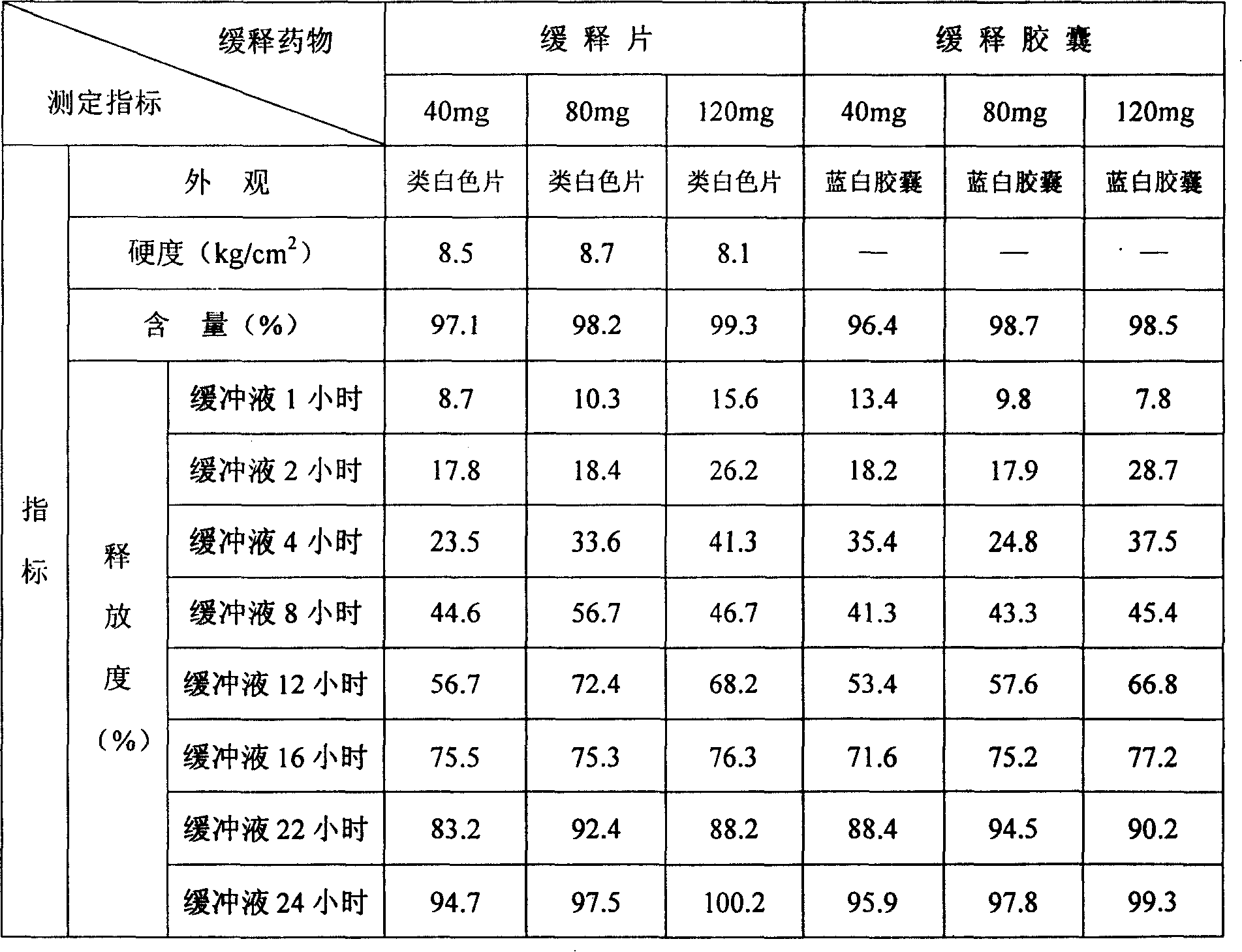
[0027] Get 15 parts of hydroxypropylmethylcellulose K15M (weight, the same below) 9.5 parts of glycine, 9.5 parts of sodium carboxymethylcellulose and 37.5 parts of lactose, pulverize, sieve, mix, and then mix with duloxyl hydrochloride Mix 28 parts of Ting, add an appropriate amount of polyacrylic resin II medicinal ethanol solution as a binder, make a soft material, granulate through a 18-mesh sieve, and obtain wet granules. The wet granules are ventilated and dried at 50°C, sieved through a 18-mesh sieve, and an appropriate amount of magnesium stearate is added, and blended to make them uniform to obtain mixed dry granules. Sampling for determination of intermediate content. Calculate the weight of the tablet to be pressed according to the determination of the intermediate content, and press it into a tablet with a φ7 deep concave die, with a hardness of 8 to 10 kg, and then coat it with an enteric-coated material to make 40 mg of duloxetine hydrochloride (take duloxetine ...
Embodiment 2
[0029] Take 13.5 parts of hydroxypropylmethylcellulose K100M, 10.5 parts of glycine, 6.5 parts of sodium carboxymethylcellulose and 26.5 parts of compressible starch, grind, sieve, mix well, and then mix with 42 parts of duloxetine hydrochloride Mix evenly, add an appropriate amount of polyacrylic acid resin II medicinal ethanol solution as a binder, make a soft material, pass through a 18-mesh sieve and granulate to obtain wet granules. The wet granules are ventilated and dried at 50°C, sized through a 18-mesh sieve, added with magnesium stearate, and blended to make them uniform to obtain mixed dry granules. Sampling for determination of intermediate content. Calculate the weight of the tablet to be pressed according to the measurement result of the intermediate content, and press it into a tablet with a hardness of 8 to 10 kg, and then coat it with an enteric-coated material to make a slow tablet containing 60 mg of duloxetine hydrochloride (calculated as duloxetine) / tablet...
Embodiment 3
[0031] Take 16 parts of carbomer 940P, 10.5 parts of glycine, 8 parts of low-substituted hydroxypropyl cellulose, and 11.5 parts of lactose, pulverize, sieve, mix well, then mix well with 55 parts of duloxetine hydrochloride, add appropriate amount Ethyl cellulose solution is used as a binder to make soft materials, and granulated through a 18-mesh sieve to obtain wet granules. The wet granules are ventilated and dried at 50°C, sized through a 18-mesh sieve, added with magnesium stearate, and blended to make them uniform to obtain mixed dry granules. Sampling for determination of intermediate content. Calculate the weight of the tablet to be pressed according to the determination result of the intermediate content, and press it into a tablet with a hardness of 8 to 10 kg, and then coat it with an enteric-coated material to make a slow tablet containing duloxetine hydrochloride 120mg (calculated as duloxetine) / tablet. release tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com