Insulin sustained-release oral preparation and preparation method thereof
A technology for sustained-release preparations and insulin, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations with inactive ingredients, etc., can solve the problems of hypertrophic lipodystrophy, subcutaneous lipoatrophy, induration and drug resistance, etc., and achieves good results. Sustained release effect, blood sugar concentration reduction, effect of broad clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
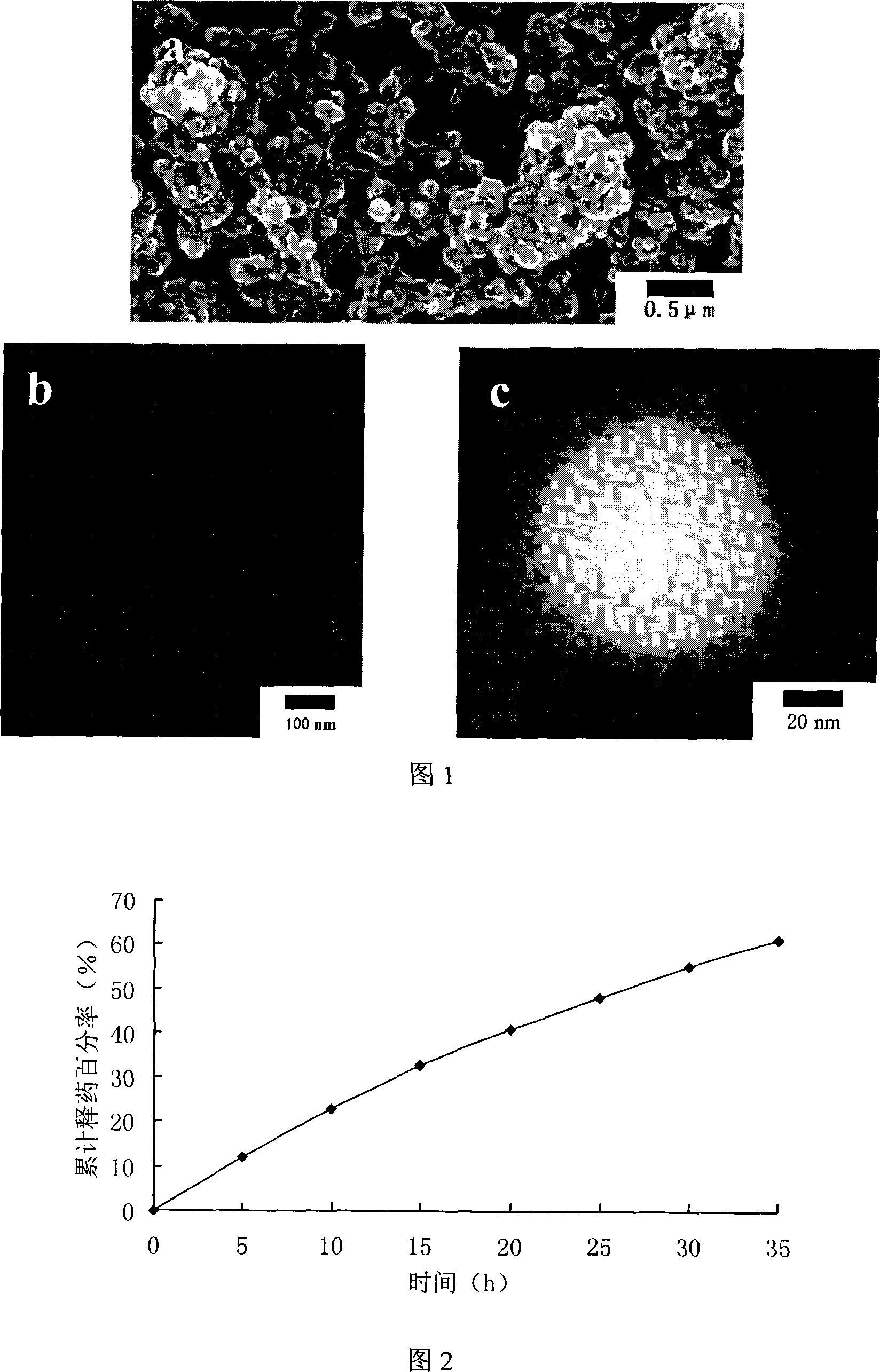
[0017] Dissolve 3 g of dragon's blood in 100 mL of ethanol solution, filter to remove impurities, and obtain dragon's blood ethanol solution (3%, w / v) for later use. Weigh insulin and add to 100mL aqueous solution (pH3.0) containing 2% (w / v) dextran-70 to make the insulin concentration 2.0IU·mL -1 , drop 0.25mL Tween-20 in it, adjust the pH with 0.01M NaOH solution until the solution turns milky white, at this time the pH is 5.3, then slowly add 2mL of Dracaena ethanol solution dropwise under constant stirring, and continue stirring After 30 minutes, a colloidal solution of brick-red dragon's blood insulin nanoparticles was obtained. Take the colloidal solution of brick red dragon's blood insulin nanoparticles and heat it at 10000r min -1 centrifuge at 4°C for 30 min, add the resulting precipitate to an acidic solution of pH 3.0, and then centrifuge under the same conditions to collect the precipitate and freeze-dry to obtain an oral sustained-release preparation of insulin w...
Embodiment 2
[0019] Dissolve 2 g of dragon's blood in 100 mL of ethanol solution, filter to remove impurities, and obtain dragon's blood ethanol solution (2%, w / v) for later use. Weigh insulin and add to 100mL aqueous solution (pH3.0) containing 2% (w / v) dextran-70 to make the insulin concentration 4.0IU·mL -1 , drop 0.25mL Tween-20 in it, adjust the pH with 0.01M NaOH solution until the solution turns milky white, at this time the pH is 5.32, then slowly add 2mL of Dracaena ethanol solution dropwise under constant stirring, and continue stirring After 30 minutes, a colloidal solution of brick-red dragon's blood insulin nanoparticles was obtained. Take the colloidal solution of brick red dragon's blood insulin nanoparticles and heat it at 10000r min -1 centrifuge at 4°C for 30 min, add the resulting precipitate to an acidic solution of pH 3.0, and then centrifuge under the same conditions to collect the precipitate and freeze-dry to obtain an oral insulin sustained-release preparation wit...
Embodiment 3
[0021] Dissolve 1.5 g of dragon's blood in 100 mL of ethanol solution, filter to remove impurities, and obtain dragon's blood ethanol solution (0.5%, w / v) for later use. Add insulin to 100ml of acidic aqueous solution (pH3.0) containing 0.4% (w / v) dextran-70 and 0.6% (v / v) Tween-20, so that the concentration of insulin is 4.0IU ml -1 ; Adjust the pH to 5.31 with 0.01M NaOH solution until the solution turns milky white. Then, under continuous stirring, slowly add 8 ml of ethanol solution (0.5%, w / v) of dragon's blood into the milky white colloid solution, and react for 10 minutes to obtain brown-red dragon's blood insulin nanoparticles colloidal solution. Take the brown-red dragon's blood insulin nanoparticle colloidal solution, and use it at 10000r·min -1 centrifuge at 4°C for 30min, add the obtained precipitate to an acidic solution of pH 3.0, and then centrifuge under the same conditions, take the precipitate, freeze-dry to obtain insulin oral sustained-release preparation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com