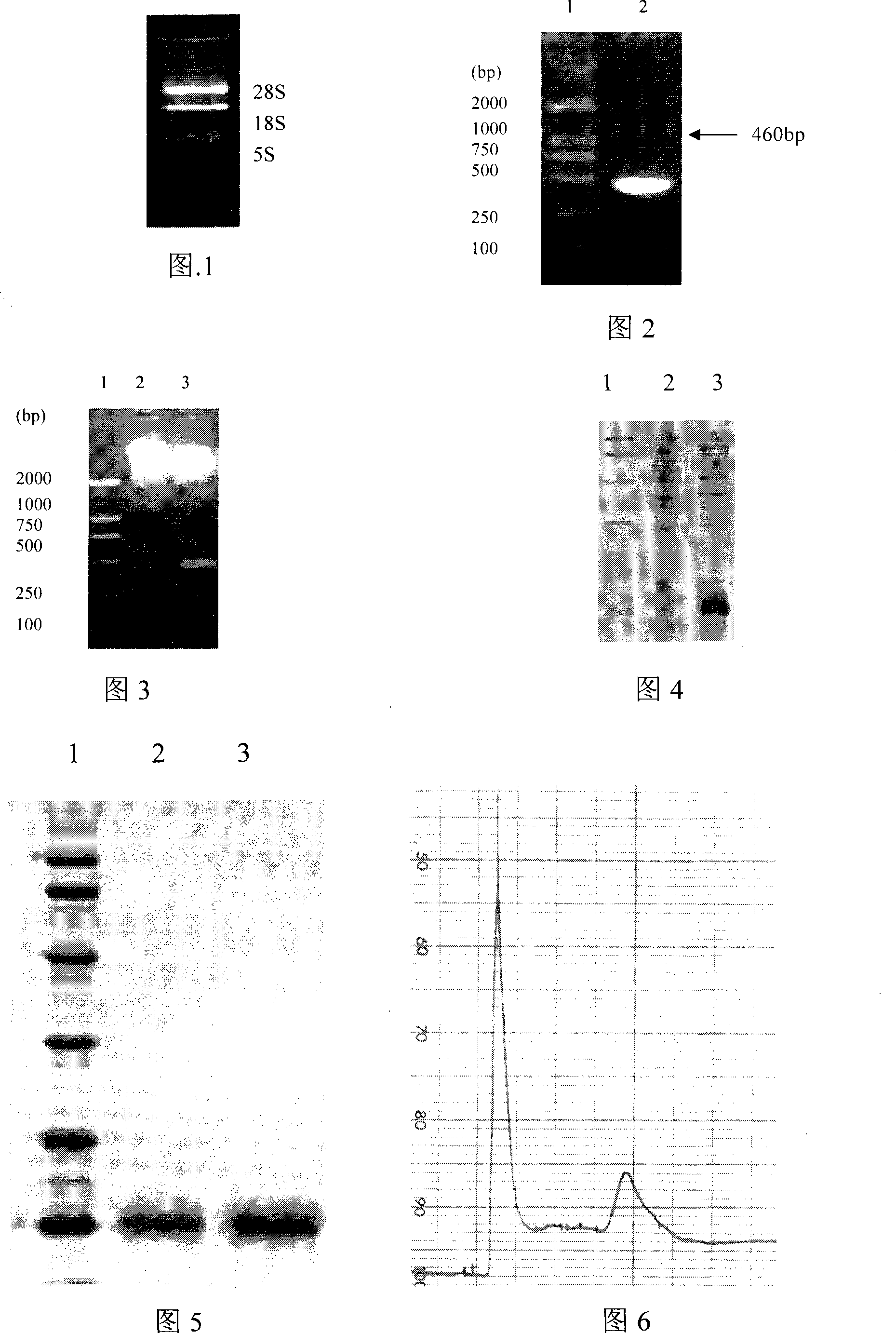
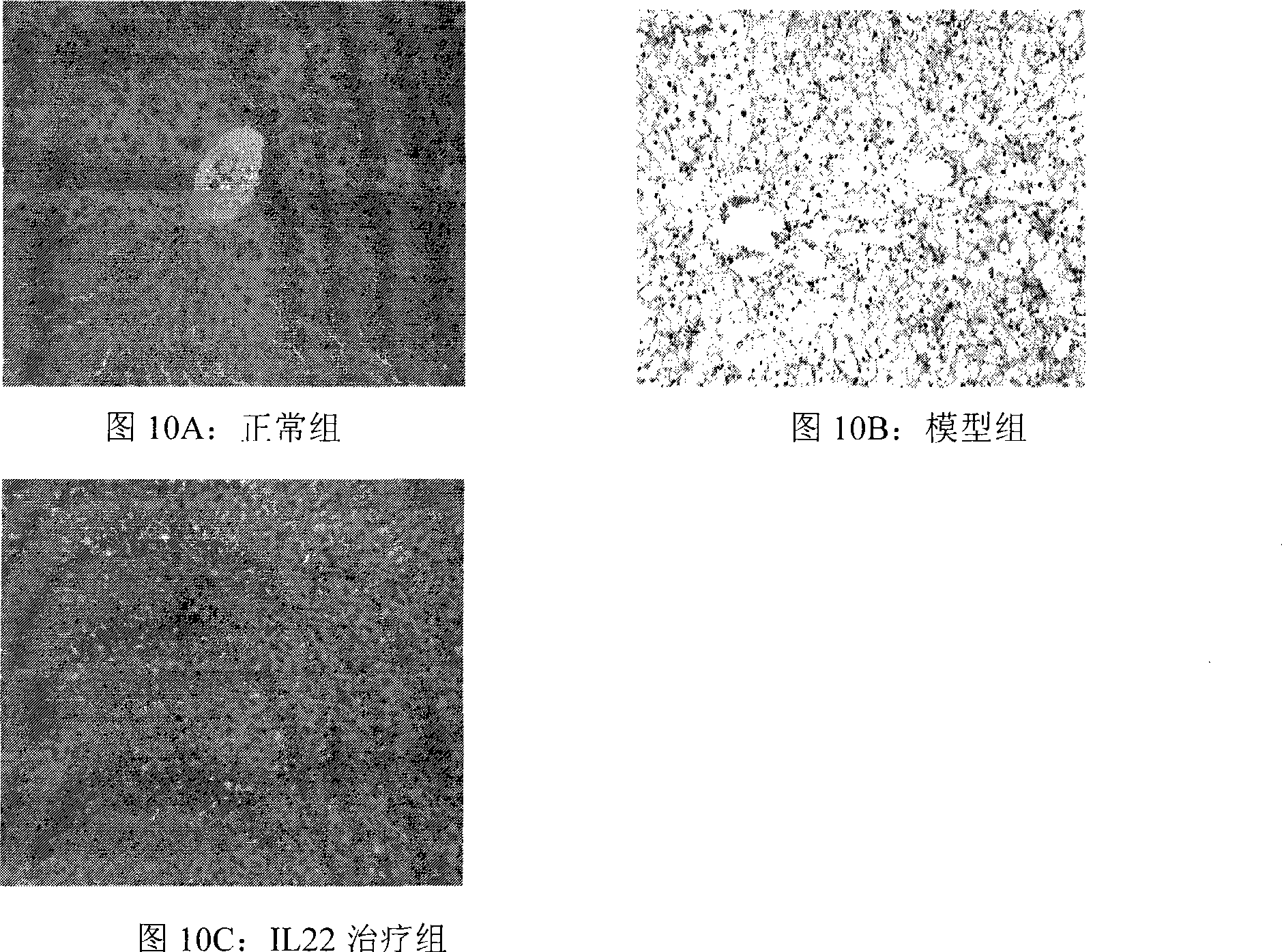
Application of interleukin-22 in preparing medicine for treating hepatopathy and preparation method thereof
A technology for interleukin and human interleukin, which is applied in the field of preparation of recombinant human interleukin-22, can solve the problems of high cost, small expression amount, low cost and the like, and achieves the effects of low production cost, high expression amount and expanding application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Preparation of hIL22
[0073] (1) Capture of hIL-22 gene and construction of recombinant expression vector
[0074] a. Extraction of total RNA Dilute 1ml of human fresh peripheral blood by 2 times with PBS containing 2mmol / L EDTA, spread it on an equal volume of lymphocyte separation medium (purchased from Shanghai Huajing Biological Co., Ltd.), and then run it at 800r / min Centrifuge to make layers, and then use capillaries to suck out the cells of the gray and white coat; wash the gray and white coat cells with Hanks solution (purchased from Tianrun Shanda Co., Ltd.) cells, and at 37°C, 5% CO 2 Cultivate in the incubator for 24 hours, and then add ConA (2 mg / ml, purchased from Xiasi Biological Company) and anti-CD respectively 3 (4mg / ml purchased from Shanghai Wowu Biotechnology Co., Ltd.) to co-stimulate the cells, and then at 37 ° C, 5% CO 2 Continue culturing in the incubator for 24 hours, and then extract the total RNA with a total RNA extraction kit (Promega Co...
Embodiment 2
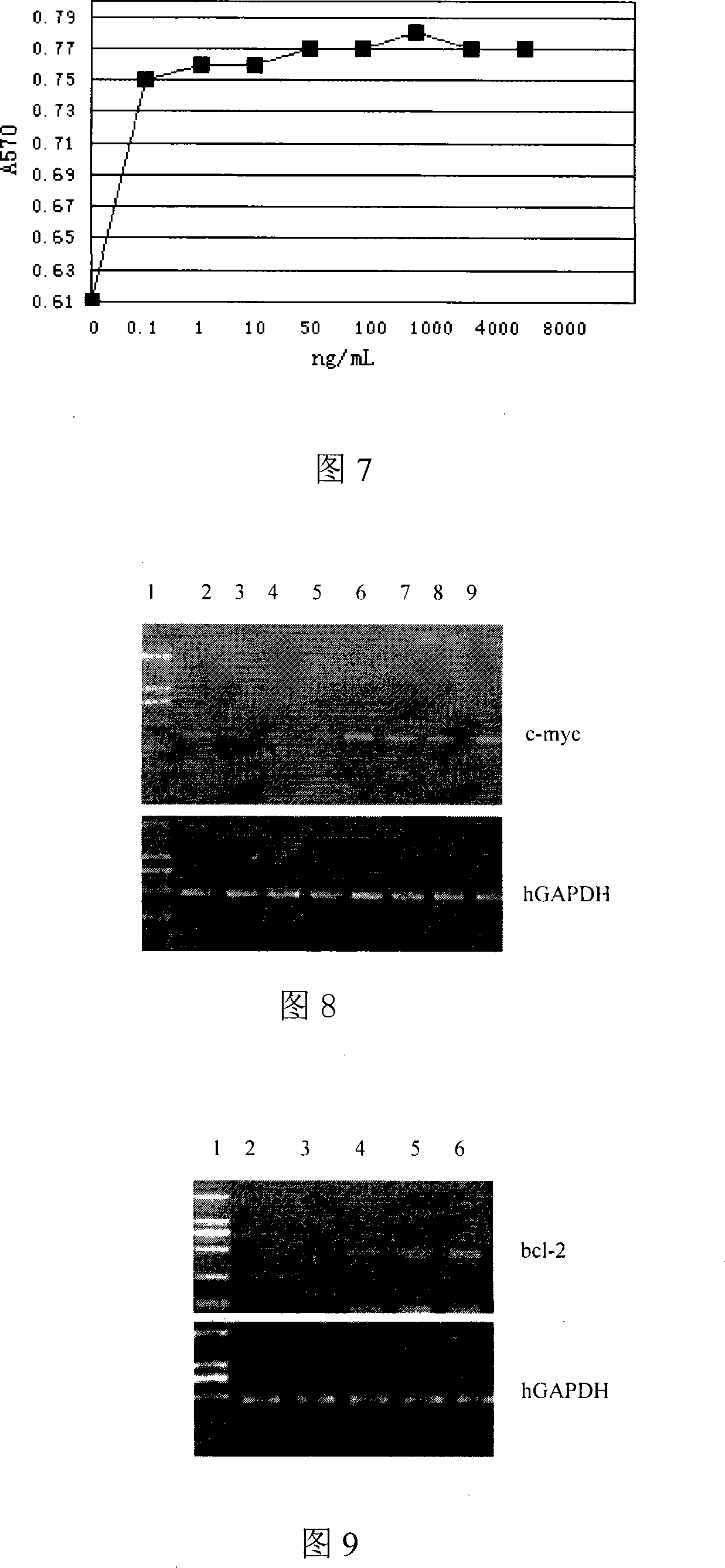
[0086] Proliferation-promoting activity of hIL22 on LO2 cells
[0087] LO2 cells were cultured with RPMI1640 culture medium containing 10% fetal bovine serum (purchased from Gibco BRL Company) until the cells grew well, and the cells were collected and divided into 1 × 10 5 cells / mL were inoculated in 96-well cell culture plates, 50 μl / well; then 50 μl of the hIL-22 protein obtained in Example 1 were added to each well, and the final concentrations were 0.1, 1, 2.5, 10, 50, 100, 1000, 2000, 4000 and 8000ng / ml, set up a blank control without cells and a negative control with cells but no factors, and do three repetitions for each concentration; at 37°C, 5% CO 2 After culturing in the incubator for 72 hours, add 20 μl of 5 mg / ml MTT to each well; continue to cultivate for 5 hours, then add 100 μl of 10% SDS (dissolved in 0.01N HCl) to each well, and measure the absorbance at 570 nm after the purple formazan crystals are dissolved value.
[0088] The concentration of hIL-22 aft...
Embodiment 3
[0090] Effect of hIL22 on the expression level of c-myc in LO2 cells
[0091] LO2 cells were cultured in a 60 mm culture dish for 2 h, and then grouped into groups and sequentially added the recombinant IL-22 protein solution prepared in Example 1 so that the final concentrations of various proteins were 20, 200, 400, and 800 ng / mL, respectively. 37°C, 5% CO 2 After culturing for 2 hours, RNA was extracted and quantitatively performed by RT-PCR. The results (see Figure 8) showed that after being stimulated by exogenous hIL-22, the expression level of c-myc gene gradually increased from 20 to 800 ng / mL, which was dependent on the concentration of hIL-22, indicating that hIL22 can increase the c - expression level of myc.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com