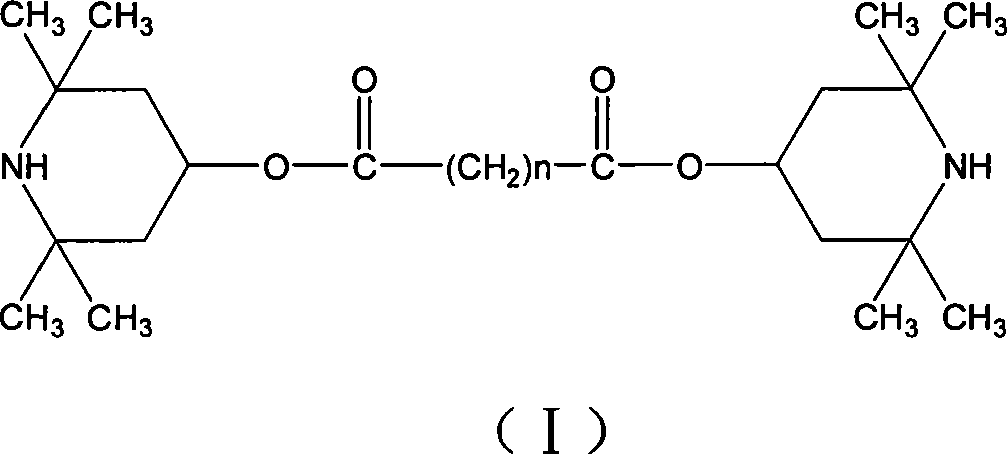
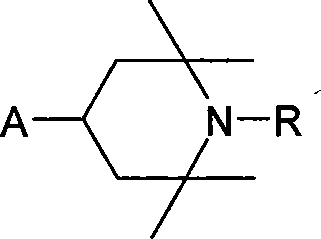
Undecane to pentadecane dicarboxylic acid di(2,2,6,6-tetramethylpiperidinyl)ester and use thereof
A technology of tetramethylpiperidinyl and dibasic acid dimethyl ester, which is applied in the field of hindered amine light stabilizer compounds, and can solve the problems of complex production process, limited application, high production cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
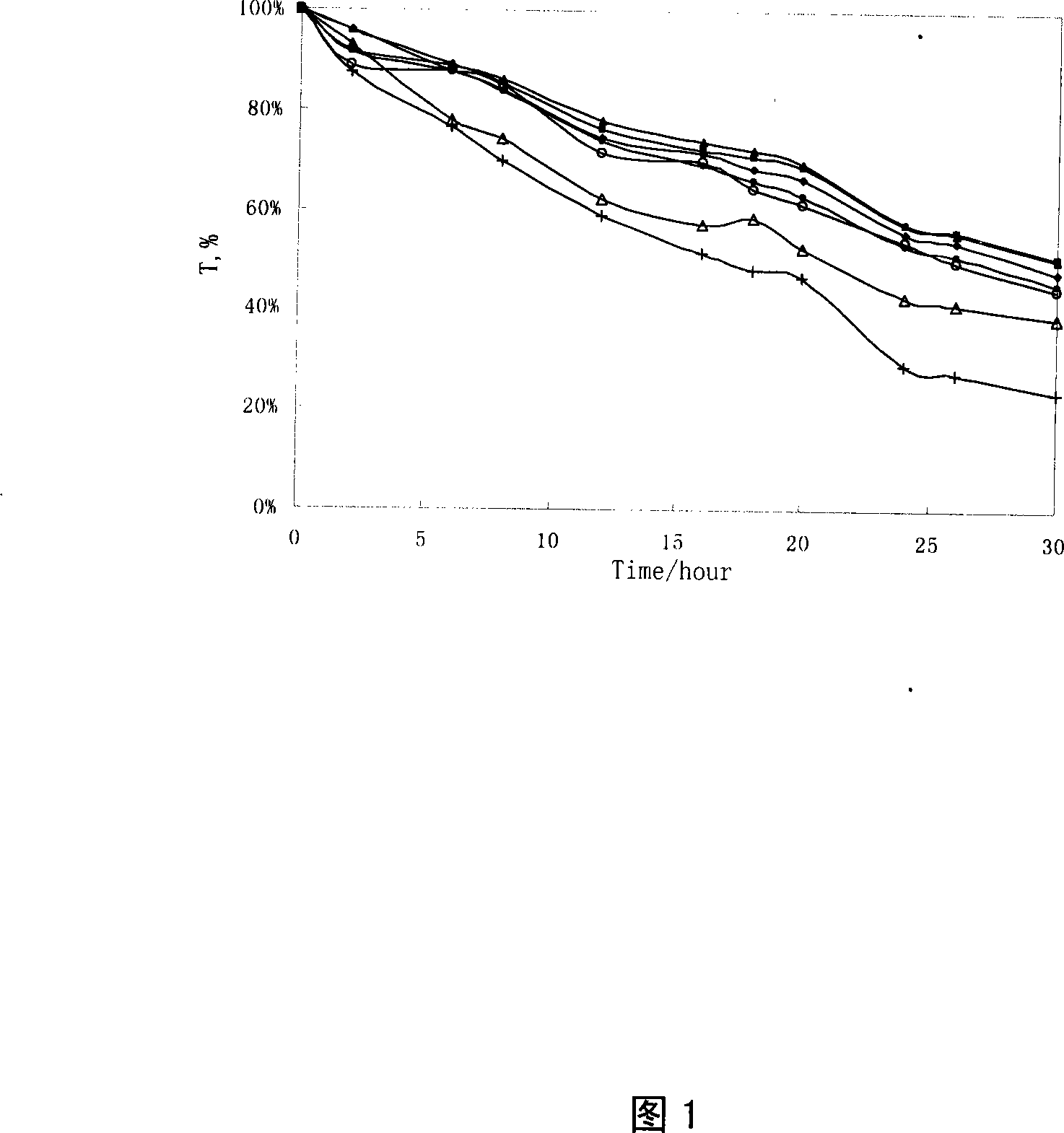
Image
Examples
Embodiment 1
[0036] Preparation of dimethyl undecanedibasic acid:
[0037] In a three-necked flask equipped with a magnetic stirrer, a thermometer, and a reflux condenser, add 0.1 mol of undecanedibasic acid, 40 ml of methanol, and 0.3 ml of catalyst concentrated sulfuric acid. After reacting for 4 hours, add 2 times the amount of methanol to the reaction solution. Petroleum ether, separate the upper layer material containing long-chain dibasic acid dimethyl ester and petroleum ether and the lower layer material containing long-chain dibasic acid, and finally carry out reflux esterification reaction on the lower layer material, and combine the reaction liquid and the extracting liquid, Wash with 50ml of 2% NaOH solution, then wash with deionized water until neutral, and distill off the solvent under reduced pressure to obtain dimethyl undecanedibasic acid in the form of light yellow liquid;
[0038] The preparation of undecane dibasic acid di(2,2,6,6-tetramethylpiperidinyl) ester:
[0039...
Embodiment 2
[0041] Preparation of undecanedibasic acid diethyl ester:
[0042] In a three-necked flask equipped with a magnetic stirrer, a thermometer, and a reflux condenser, add 0.1 mol of undecanedibasic acid, 50 ml of ethanol, and 0.4 ml of catalyst concentrated sulfuric acid. After reacting for 5 hours, add 2 times the amount of ethanol to the reaction solution Petroleum ether, separate the upper layer material containing long-chain dibasic acid diethyl ester and petroleum ether and the lower layer material containing long-chain dibasic acid, and finally carry out the reflux esterification reaction of the lower layer material for 4 hours, and combine the reaction liquid and the extract , washed with 50ml of 2% NaOH solution, then washed with deionized water until neutral, distilled under reduced pressure, and evaporated the solvent to obtain diethyl undecanedibasic acid in the form of light yellow liquid.
[0043] The preparation of undecane dibasic acid di(2,2,6,6-tetramethylpiperid...
Embodiment 3
[0046] Preparation of Dimethyl Dodecanedibasic Acid
[0047] In a three-necked flask equipped with a magnetic stirrer, a thermometer, and a reflux condenser, add 0.1 mol of dodecanedibasic acid, 40 ml of methanol, and 0.3 ml of catalyst concentrated sulfuric acid. After reacting for 4.5 hours, add an amount equivalent to 4 Double the petroleum ether, separate the upper layer material containing long-chain dibasic acid dimethyl ester and petroleum ether and the lower layer material containing long-chain dibasic acid, and finally carry out the reflux esterification reaction on the lower layer material, and combine the reaction liquid and the extract , wash with 50ml of 2% NaOH solution, then wash with deionized water to neutrality, and distill off the solvent under reduced pressure to obtain light yellow liquid dimethyl dodecanedioic acid;
[0048] Preparation of dodecanedioic acid di(2,2,6,6-tetramethylpiperidinyl) ester:
[0049] A thermometer and a Soxhlet extractor equipped...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com