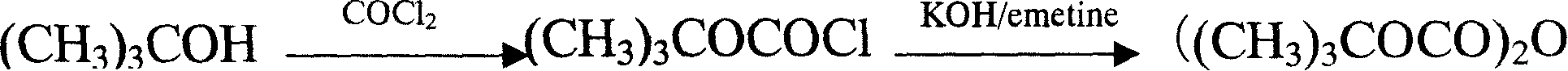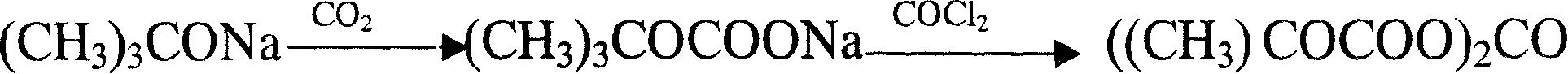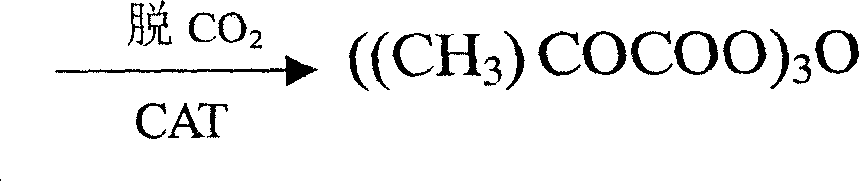Method for synthesizing di-tert-butyl dicarbonic acid ester
A technology of di-tert-butyl dicarbonate and synthesis method, which is applied to the preparation of carbon dioxide or inorganic carbonate, organic chemistry, reagents, etc., and can solve the problems of large specificity of methanesulfonyl chloride, reduced reactivity, and difficult separation of products , to achieve the effects of easy separation, improved solubility, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 400g (460ml) of toluene to 50g (0.52mol) sodium tert-butoxide, heat to 60°C to dissolve sodium tert-butoxide, cool down to 50°C, feed carbon dioxide at a flow rate of 0.2-0.3L / min, until the solution is flooded White, about 2.5hr; add 100g of tert-butanol; add catalyst N,N,N',N'-tetramethylethylenediamine (TMEDA) 0.15g, cool down to 0-20℃, drop diphosgene (Cl 3 COCOCl) 27g (0.136mol) of toluene solution 80 grams, add dropwise for 2-3 hours, continue to stir for 2.5 hours after the end, add 100 grams of anhydrous sodium carbonate solution for alkali washing, 150 grams of citric acid solution for acid washing, and 100 grams of water for washing When the pH of the washing water was equal to 5-7, the reaction liquid was distilled under reduced pressure to obtain 47.51 g of DIBOC with a yield of 83.73%.
Embodiment 2
[0039] Add 400g (238ml) of heptane to 50g (0.52mol) of sodium tert-butoxide, heat to 60°C to dissolve sodium tert-butoxide, cool down to 50°C, and feed carbon dioxide at a flow rate of 0.2-0.3L / min for 2.5hr; Supplement 100g (116ml) of toluene, 90g of tert-butanol, add 0.06g of catalyst triethylenediamine, lower the temperature to 0-20°C, add diphosgene (Cl 3 COCOCl) 25g (0.126mol) of toluene solution 80g, stirred for 2.5 hours, washed with anhydrous sodium carbonate alkali, washed with water until the washing water pH4.63, conductivity 24.6μs, the reaction solution was distilled under reduced pressure to obtain DIBOC43.19g, yield 76.12 %.
Embodiment 3
[0041] Add 400g (460ml) of toluene to 50.3g (0.52mol) sodium tert-butoxide, heat to 60°C to dissolve sodium tert-butoxide, cool down to 50°C, feed carbon dioxide at a flow rate of 0.2-0.3L / min, 2.5hr; Tetrahydrofuran 150ml; add catalyst N,N,N',N'-tetramethylethylenediamine 0.2g, cool down to 0-20°C, add diphosgene (Cl 3 COCOCl) 28.9g (0.146mol) of toluene solution 80g, stirred for 2.5 hours, washed with water until the pH of the washing water was 5.04, the conductivity was 77.1μs, and the reaction solution was distilled under reduced pressure to obtain 44.66g of DIBOC with a yield of 78.71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com