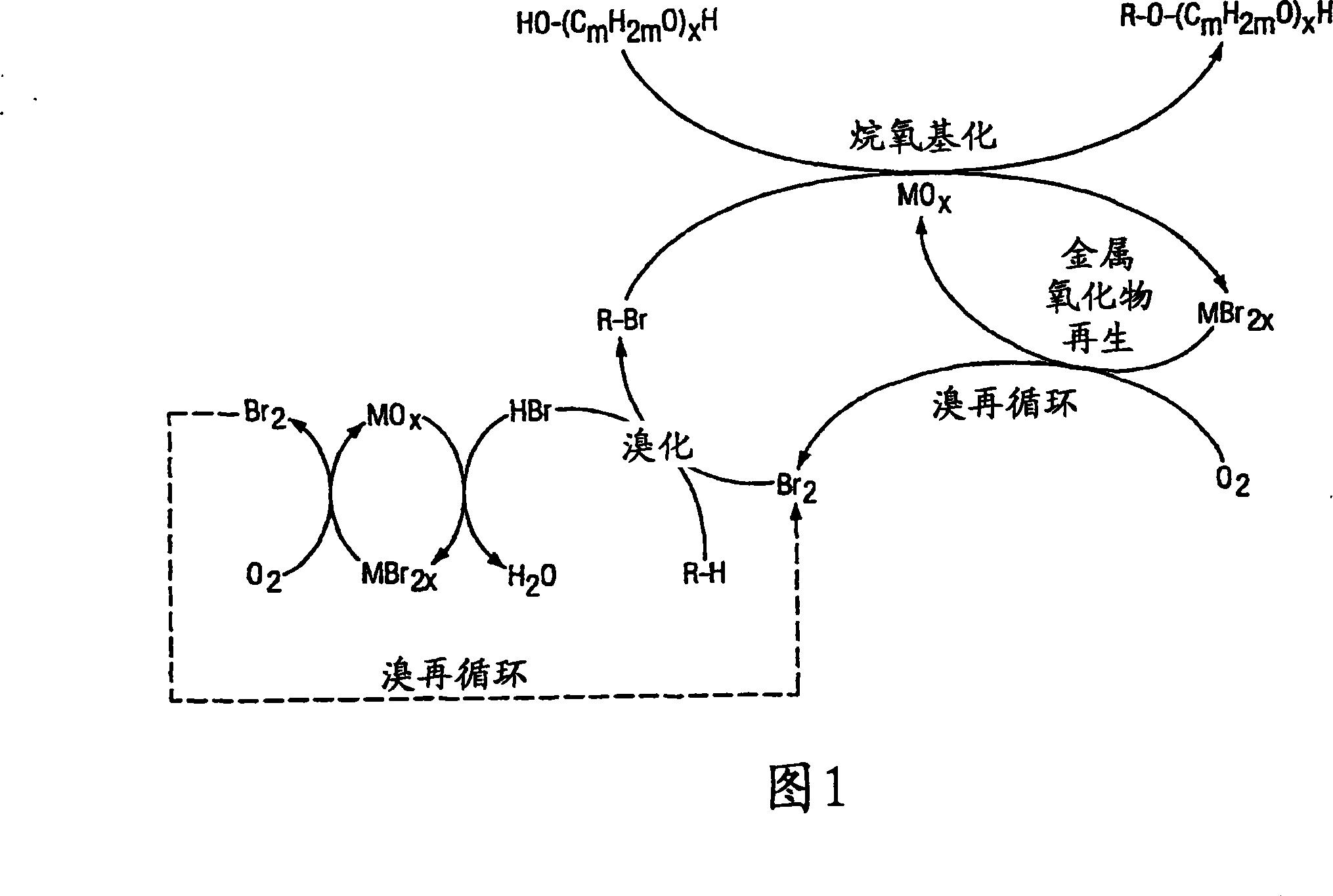
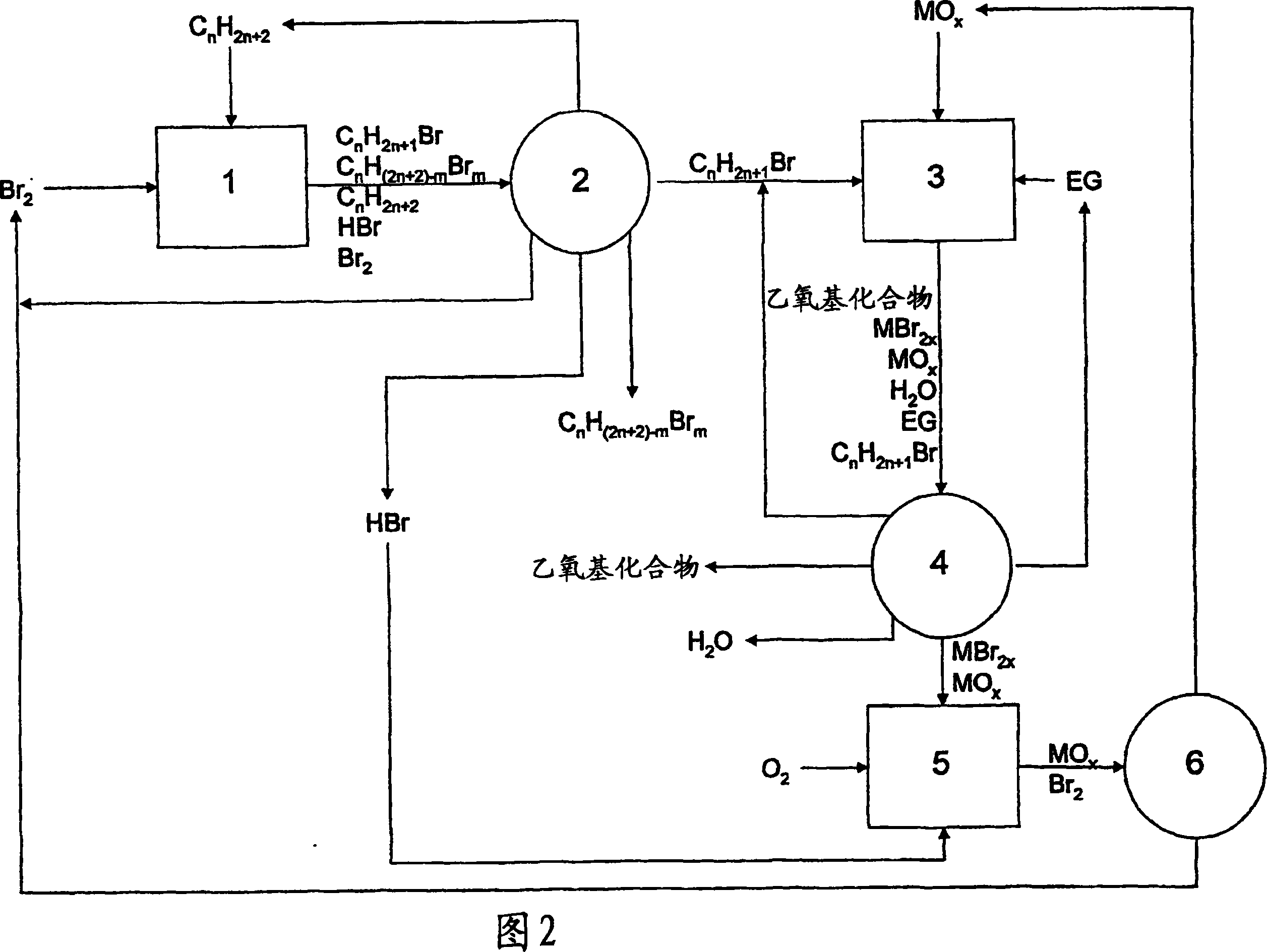
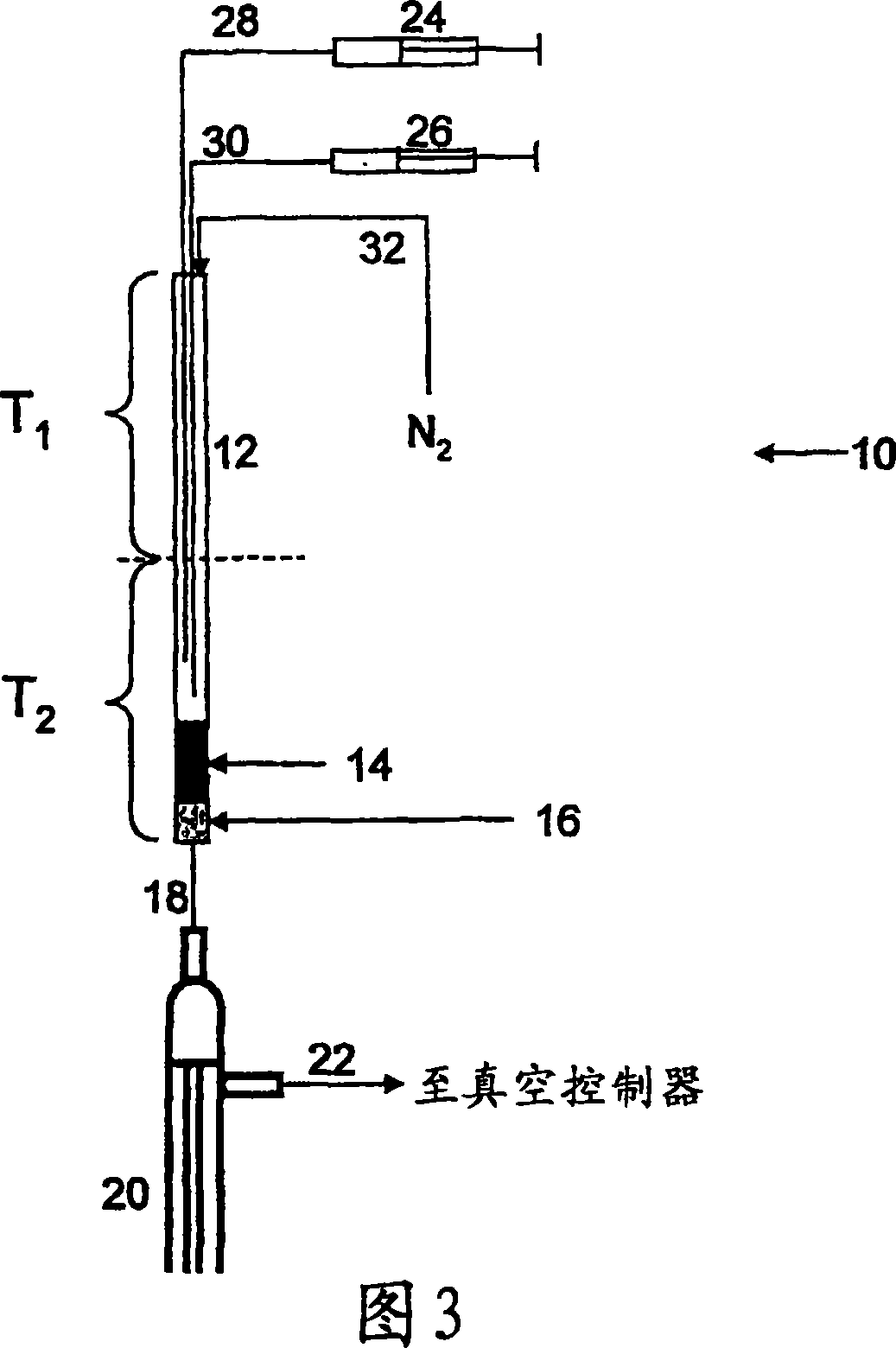
Method of making alkoxylates
A compound and a technology for producing alkanes, applied in sustainable manufacturing/processing, ether preparation, organic chemistry, etc., can solve problems such as volatility instability, concentration, and non-optimal product properties of ethylene oxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] An approximately 3 mL stainless steel batch reactor was filled with 0.2549 grams of electronic grade magnesia (eMgO) and 0.2543 grams of a solution of 75 wt% 2-bromododecane and 25 wt% octadecane (as internal standard). Stir with a stainless steel spatula to mix the solids and liquids, then add 0.3065 grams of ethylene glycol (EG). The reactor was sealed and agitated with a vibrating shaker for 5 minutes, then placed in a preheated oven at 225°C for 6 hours. Once cooled, the organics were extracted with ethanol and analyzed by gas chromatography and mass spectrometry to characterize and quantify the product and starting material. Analysis showed that 49% of the 2-bromododecane was converted to product. The product consisted of 56% olefins, 3% alcohols, 40% monoethoxylates and 1% ketones.
Embodiment 2
[0084] An approximately 3 mL stainless steel batch reactor was filled with 0.2531 g of copper(II) oxide (CuO) and 0.2500 g of a solution of 75 wt% 2-bromododecane and 25 wt% octadecane (as internal standard). Stir with a stainless steel spatula to mix the solids and liquids, then add 0.0976 grams of EG. The reactor was sealed and agitated with a vibrating shaker for 5 minutes, then placed in a preheated oven at 225°C for 6 hours. Once cooled, the organics were extracted with ethanol and analyzed by gas chromatography and mass spectrometry to characterize and quantify the product and starting material. Analysis showed 97% conversion of 2-bromododecane to product. The product consists of 58% olefins, 9% alcohols, 32% monoethoxylates and 1% ketones.
Embodiment 3
[0086] An approximately 3 mL stainless steel batch reactor was filled with 0.2501 g of copper(II) oxide (CuO) and 0.2538 g of a solution of 75 wt% 2-bromododecane and 25 wt% octadecane (as internal standard). Stir with a stainless steel spatula to mix the solids and liquids, then add 0.1002 grams of EG. The reactor was sealed and agitated with a vibrating shaker for 5 minutes, then placed in a preheated oven at 225°C for 3 hours. Once cooled, the organics were extracted with ethanol and analyzed by gas chromatography and mass spectrometry to characterize and quantify the product and starting material. Analysis showed that 42% of the 2-bromododecane was converted to product. The product consisted of 31% olefins, 5% alcohols, 63% monoethoxylates and 1% ketones.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com