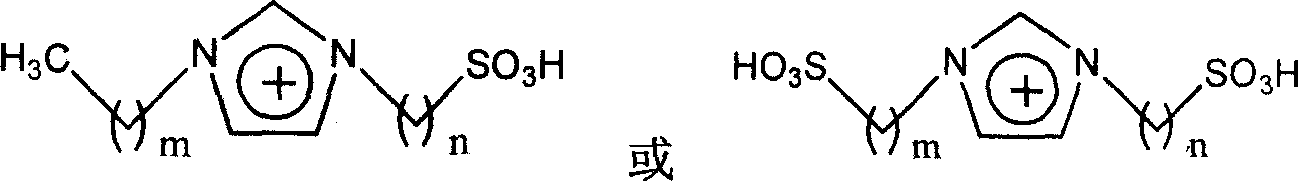
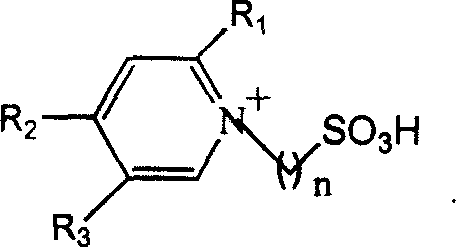
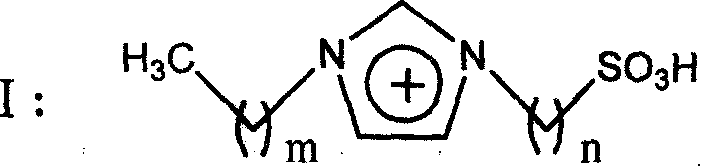
Method for alkylation of isoparaffin and olefin catalyzed by ion liquid
A technology of isoparaffin and acidic ionic liquid, which is applied in the field of acidic ionic liquid catalyzing the alkylation reaction of isoparaffin and olefin, can solve the problems of difficult separation of oil and catalyst, strong corrosiveness, and strict requirements on water content, etc. The catalyst can be recycled, the reaction conditions are mild, and the catalyst dosage is small.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 3.0 g of ionic liquid a and 100 mL of water into a 250 mL high pressure reactor. Close the reactor, evacuate most of the air, and use a certain pressure of N 2 The reactor was replaced three times. The weighing method is to enter 80g of mixed gas of isobutane and butene-1, and the ratio of alkanes to olefins is 6. Start stirring and heat. Increase the temperature to 60°C within 30min, fill with N 2 To 1.6MPa, keep the reaction at 60°C / 1.6MPa for 0.5h. After the reaction, cool to room temperature and release the pressure. The reaction solution was poured into a separatory funnel, and allowed to stand to separate into layers. The lower layer was a colorless and transparent water phase, and the upper layer was a light yellow and transparent oil phase to obtain 4.5 g of the oil phase. Chromatographic analysis, quantified by normalization method. The reaction conversion rate of butene-1 is 20.5%, the yield of alkylated oil is 54.7%, and the composition of alkylated...
Embodiment 2~13
[0033] With embodiment 1, reaction condition and result are shown in table 1 and table 2.
[0034] Table 1 Embodiment 2~13 test conditions
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com