Novel steroid compound and uses thereof
A compound and reaction technology, applied in the field of [17α, can solve the problems of easy explosion, high risk, low yield, etc., and achieve the effect of high safety performance and operability, excellent yield and cost, and simple circuit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Condensation, ring opening reaction
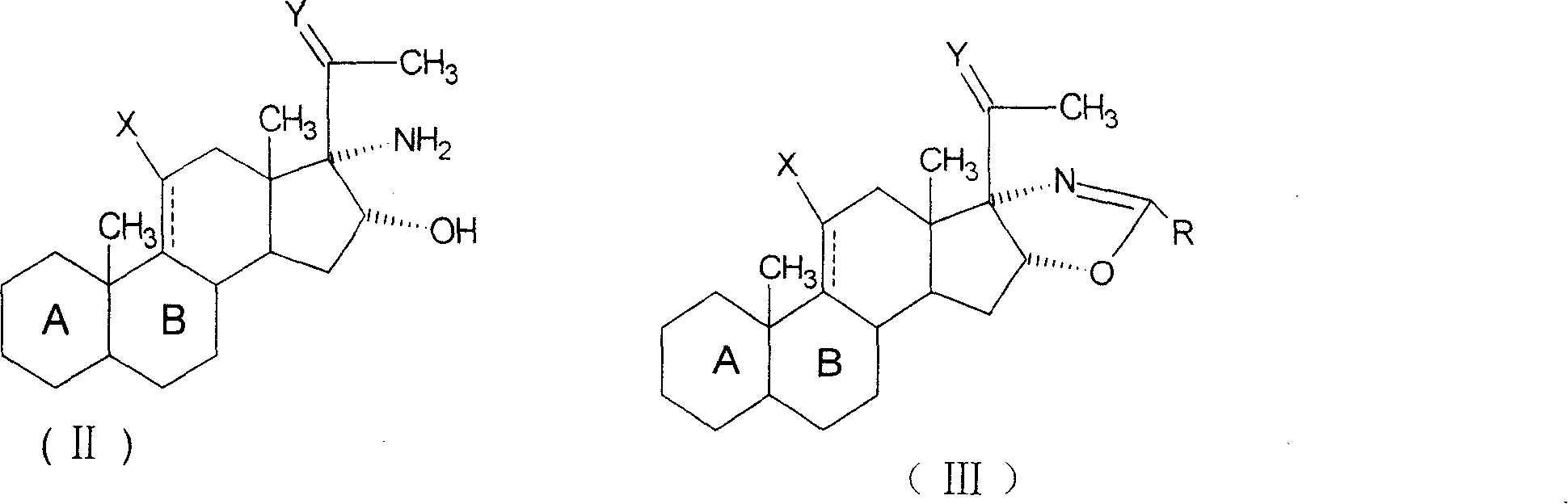
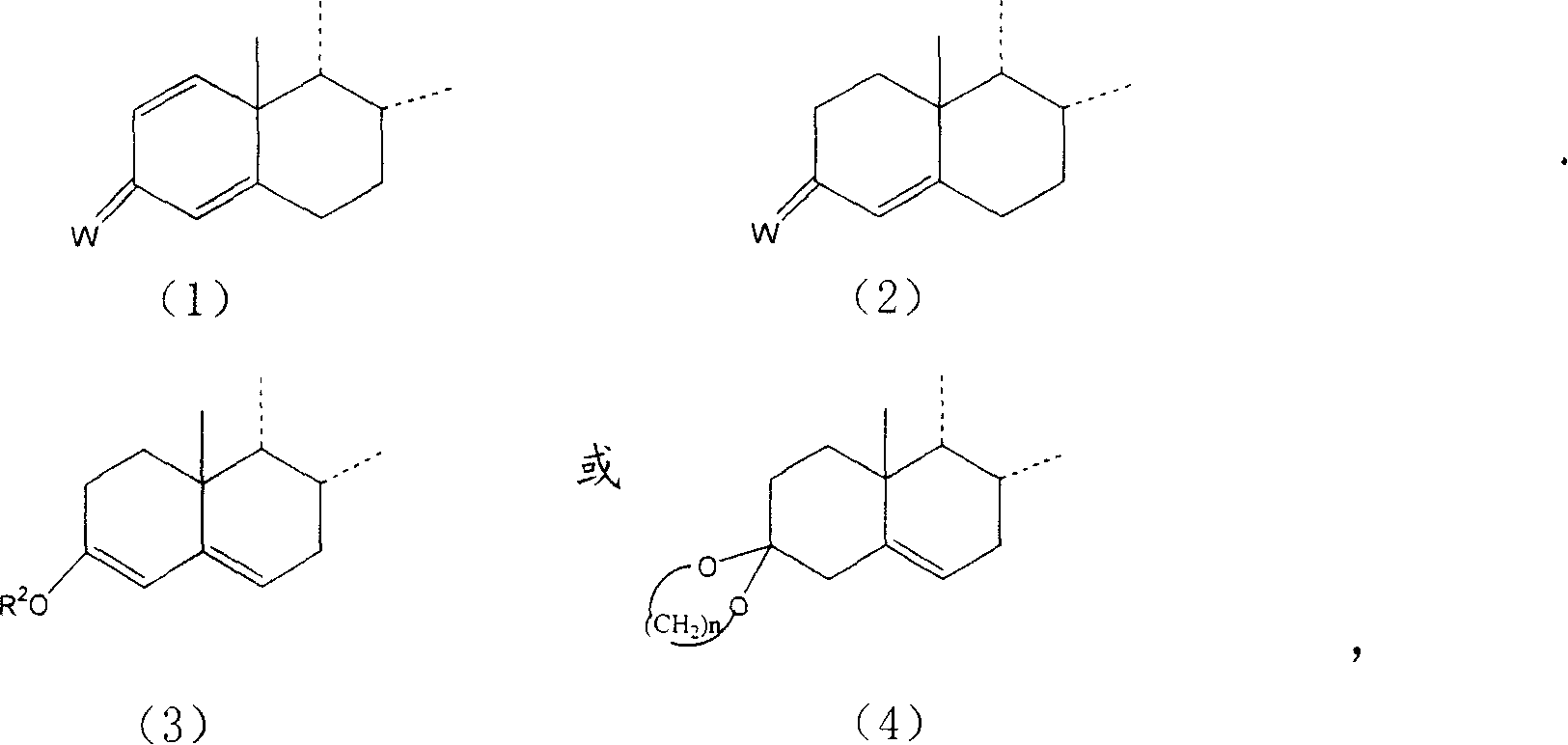
[0048] Add 32.4g of 1,4,9-triene-16,17-epoxy-3,20-diketone pregna (ZL200210014479.5) and 205ml of tetrahydrofuran into the reaction flask. After replacing the air with nitrogen, add Semicarbazide 20.8gH 2 NNH CONH 2 , 0.205g p-toluenesulfonic acid, temperature control 20-25℃, react for 2 hours, take samples for TLC analysis until there is no raw material, change to ammonia gas, the temperature is 20-25℃, stop the gas conduction after 3 hours, and then react for 1 hour , thin-layer analysis to no raw materials, exhaust the ammonia with atmospheric nitrogen, dilute the reaction solution in 1200ml of saturated sodium chloride water, stir for 1 hour, let stand for 1 hour, filter, and dry to obtain 34g of compound 16α-hydroxyl- 17α-Amino-1,4,9-trienpregna-3-one-20-semicarbazone.
[0049] Cyclization reaction
[0050] Add 30g of 16α-hydroxy-17α-amino-1,4,9-triene-pregn-3-one-20-semicarbazone, 150ml of acetic anhydride, 150ml of acetic...
Embodiment 2
[0056] Condensation, ring opening reaction
[0057] Add 30g of 16,17-epoxy-11-hydroxyl-1,4-dienepregna-3,20-dione (ZL200210014479.5), 180ml of dimethylformamide into the reaction flask, and feed nitrogen After replacing the air, add semicarbazide 18gH 2 NNH CONH 2 , 0.18g p-toluenesulfonic acid, react at a temperature of 25-30°C for 2 hours, take a sample for thin-layer analysis until there is no raw material, change to ammonia gas, the temperature is 10-15°C, stop the gas conduction after 5 hours, and then react for 1.5 hour, thin-layer analysis to no raw materials, exhaust the ammonia with atmospheric nitrogen, dilute the reaction solution in 800ml of saturated sodium chloride water, extract three times with 150ml of chloroform, combine the organic phases, wash with water until neutral, concentrate under reduced pressure, Replace with ethyl ester 2 to 3 times when the volume reaches a small size, and the precipitated material is cooled at 0 to 5°C for 1 hour, filtered and ...
Embodiment 3
[0065] Synthetic Deflazacort:
[0066] The 2′-methyl-5′βH-pregna-1,4,9-triene-[17α,16α-d]-oxazole-3,20-dione obtained in Example 1 was used as the raw material.
[0067] 1) Ethylene bond addition reaction:
[0068] Add 18.3g of 2′-methyl-5′βH-pregna-1,4,9-triene-[17α,16α-d]-oxazole-3,20-dione and 183ml of tetrahydrofuran into the reaction flask, After dissolving, cool down to 0±5°C, quickly add 110ml of 4.8% perchloric acid aqueous solution dropwise, then add 22g of N-bromosuccinimide, react for 30 minutes, take samples for lamellar analysis to determine the end point, add 30ml of saturated sodium sulfite The aqueous solution is discolored by reaction. After stirring for 30 minutes at 10±2°C, pour the reaction solution into 1200ml of saturated sodium chloride ice water for dilution, stir for 1 hour, let stand for 1 hour, filter, wash with water until neutral, and dry to obtain 22g of the compound 11-Hydroxy-9-bromo-2'-methyl-5'βH-pregna-1,4,9-triene-[17α,16α-d]-oxazole-3,20-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com