H3 type flu virus hemagglutinin space conformation simulation antigen epitope and application thereof
A technology for antigenic epitopes and influenza virus, applied in the field of biomedicine, can solve the problems of inability to obtain spatial conformational epitopes, time-consuming and labor-intensive, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1, Prokaryotic expression of influenza virus hemagglutinin fragment HA1
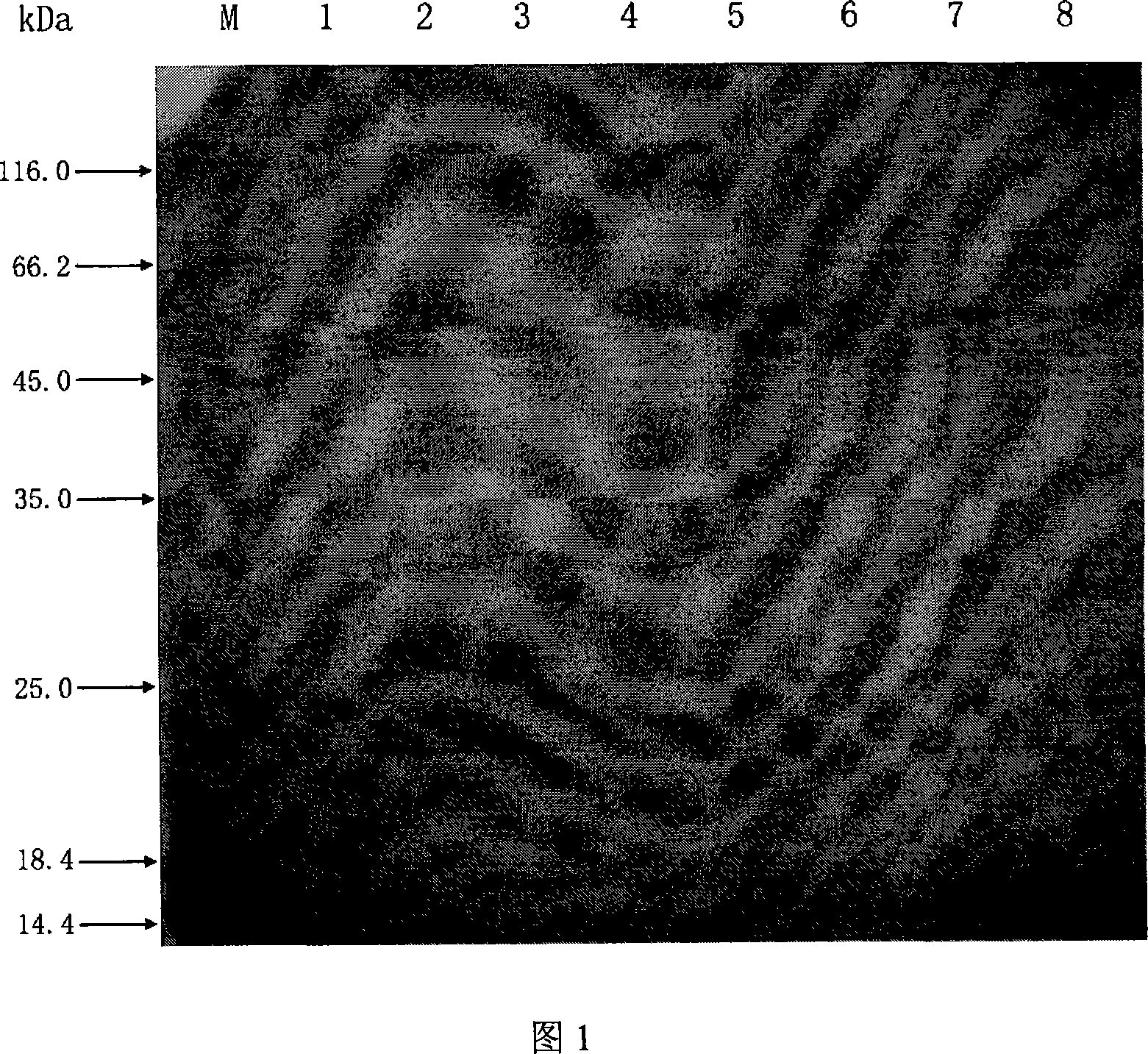
[0046] 1) Cloning of influenza virus hemagglutinin fragment HA1 gene ha1: On MDCK cells growing into a single layer, inoculate H3 influenza virus A / Swine / Guangdong / 6 / 2004 (H3N2) strain, 37 ° C 5% CO 2 Culture in an incubator for 48 hours. When obvious cell lesions appear, the cells are collected, the cells are lysed by repeated freezing and thawing, centrifuged to remove the precipitate, and the supernatant is taken. Total RNA was extracted with Trizol reagent (product of Invitrogen Company), and a pair of primers were designed according to the hemagglutinin gene sequence of influenza virus A / Swine / Guangdong / 6 / 2004 (H3N2) strain (Gene ID: AY852277), followed by reverse transcription RT-PCR was carried out under the action of enzyme (product of Invitrogen Company) and Taq enzyme (product of Takara Company) to obtain the ha1 gene, which was identified by DNA sequencing. The two primer seque...
Embodiment 2
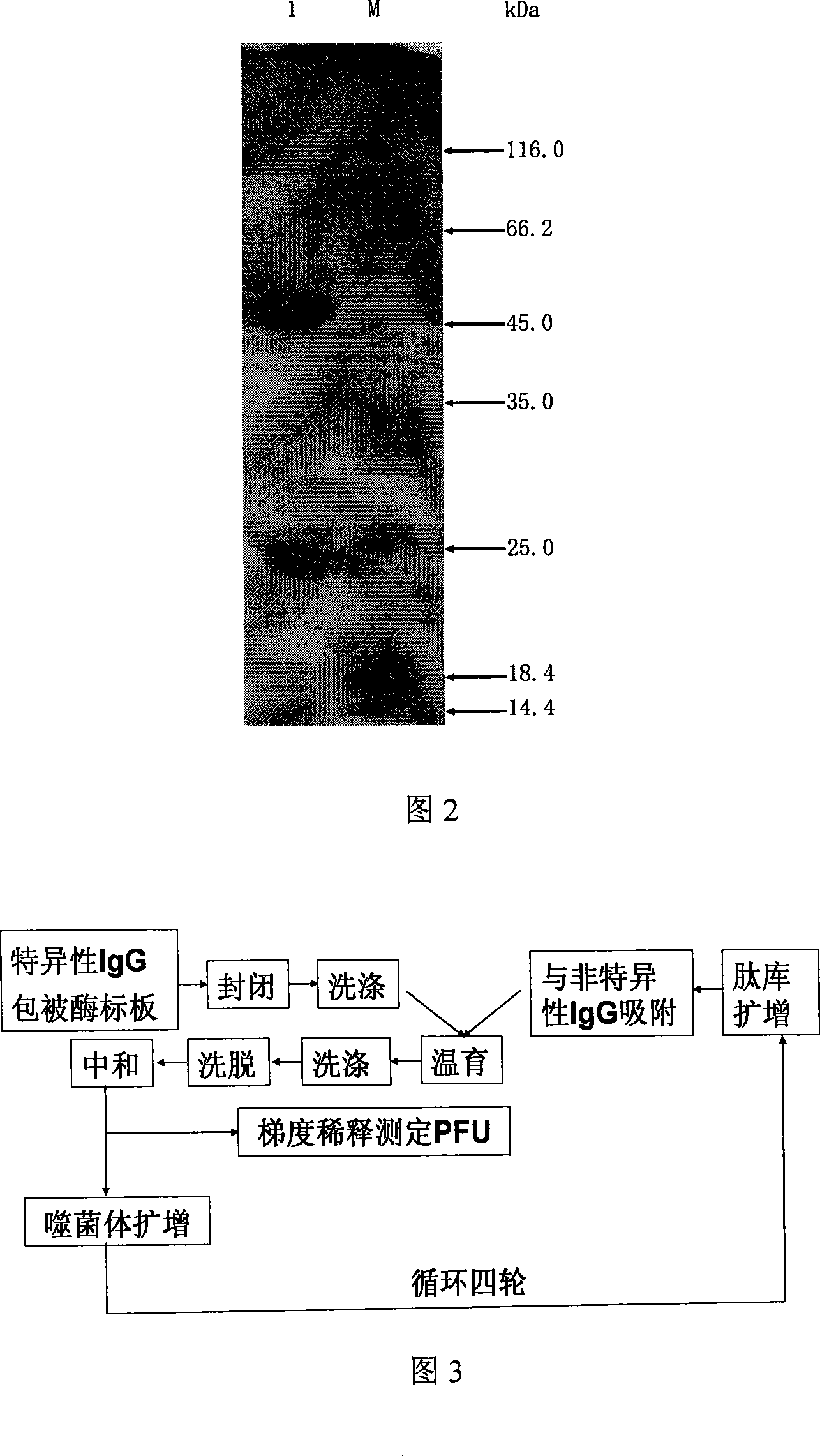
[0051] Example 2, Purification and Identification of Anti-recombinant HA1 Rabbit Serum IgG
[0052] 1) Purified recombinant HA1 was used to immunize New Zealand rabbits: the purified recombinant HA1 was adjusted to 200 μg / mL with 0.01 M, pH 7.4 PBS, mixed with an equal volume of Freund's adjuvant, emulsified, and subcutaneously multi-pointed on the back to immunize New Zealand rabbits For rabbits, the second immunization is performed two weeks after the first immunization, and the third immunization is performed two weeks after the second immunization. The second and third doses were emulsified with an equal volume of Freund's incomplete adjuvant, and were injected subcutaneously at multiple points on the back. After three immunizations, the titer of the antibody was tested. When the titer reached 1:32, blood was collected from the heart, and the serum was separated.
[0053] 2) Purification of anti-recombinant HA1 rabbit serum IgG: use saturated ammonium sulfate fractional p...
Embodiment 3
[0054] Embodiment 3, phage random peptide library screening
[0055] 1) The phage random peptide library is the M13 phage twelve peptide library (Ph.D.-12TMPhage Display Peptide Library Kit) commercialized by BioLabs
[0056] 2) Screening method: Coat the ELISA plate with anti-recombinant HA1 rabbit serum IgG (100 μg / mL) diluted in the coating solution (carbonate buffer at pH 9.6), and add blocking solution (3% BSA) at 4°C for 2-3 hours, wash with TBST, and spin dry the washing liquid. Add diluted phage random peptide library to each well, and the number of phage added in each round should be 2×10 10-11 CFU, incubate at room temperature for 1 hour, shake off the liquid, rinse with TBST buffer containing 0.1% tween-20 (v / v), and finally use 100 μl pH 2.2 glycine buffer to elute the IgG that specifically binds to anti-recombinant HA1 rabbit serum phage, and immediately neutralized with 10 μl pH 9.8 Tris buffer, 10 μl was taken for phage titer determination, and the remaining p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com