Method for constructing replication defect type recombination adenovirus
A recombinant adenovirus, replication-deficient technology, applied in the field of biomedicine, can solve problems such as weakening the therapeutic effect, and achieve the effect of wide clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
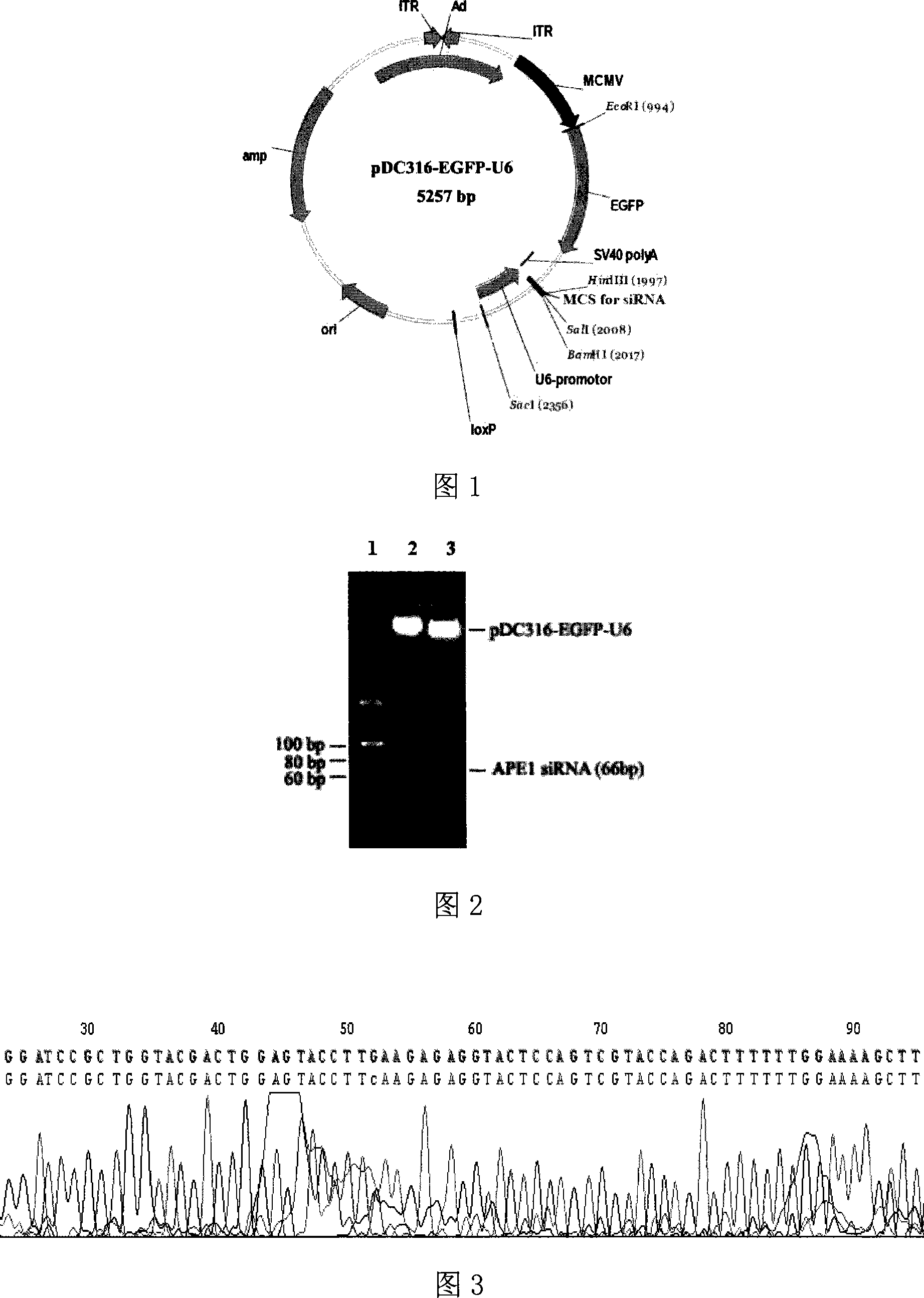
[0040] Example 1 Construction and Identification of APE1 siRNA Adenovirus Shuttle Plasmid
[0041] According to the effective human APE1 siRNA sequence designed by us [4], the Ambion siRNA target sequence analysis and design system was used to scan the cDNA coding sequence of the human APE1 gene with the gene number NM_001641. According to the siRNA target sequence selection design principle, the BLAST sequence homology After the analysis, select the specific siRNA target sequence of 867-885 in the human APE1 gene cDNA coding sequence, and design the expression sequence of the human APE1 gene siRNA. , HindIII restriction site sticky end, chemical synthesis of the following two complementary single strands:
[0042] Top strand:
[0043] 5′-GATCCGCTGGTACGACTGGAGTACCTTTCAAGAGAGGTACTCCAGTCGTACCAGACTTTTT
[0044] TTGGAAA-3';
[0045] Bottom strand:
[0046] 5′-AGCTTTTCCAAAAAAGTCTGGTACGACTGGAGTACCTCTCTTGAAGGTACTCCAGTCGT
[0047] ACCAG CG-3′
[0048] The aforementioned compleme...
Embodiment 2
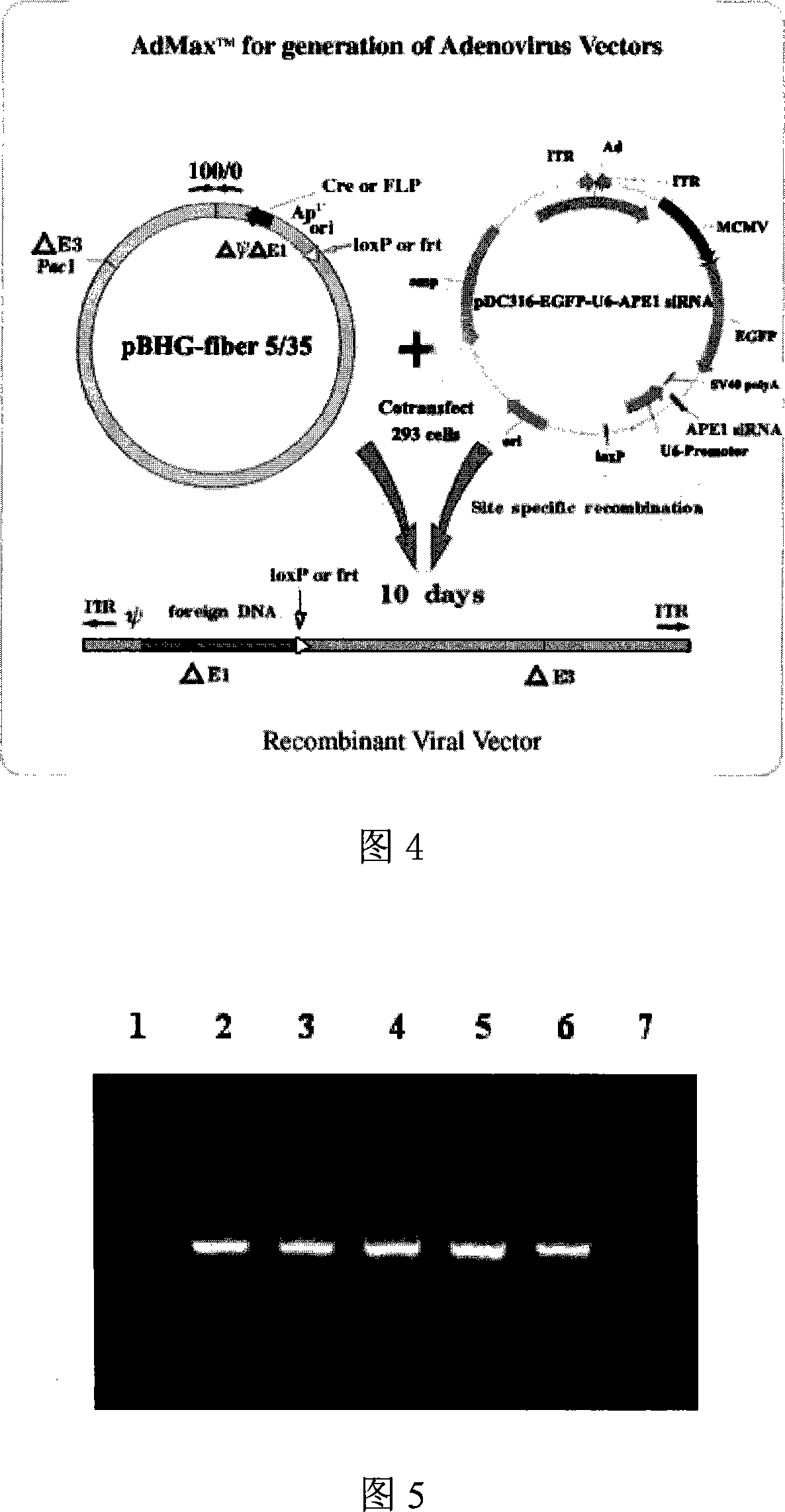
[0049] Example 2 Ad5 / F35-APE1siRNA recombinant adenovirus packaging, amplification and purification 2.1 Ad5 / F35-APE1siRNA recombinant adenovirus packaging
[0050] Packaging principle and flow chart: The recombinant adenovirus is constructed using the adenovirus Ad5 / F35MaxTM packaging system of Benyuan Zhengyang Company. The shuttle plasmid pDC316-EGFP-U6-APE1siRNA carrying the human APE1 gene siRNA expression sequence and the adenovirus backbone plasmid pBHG-fiber5 / 35 were co-transfected into 293 cells. Plasmids are combined, and Cre / loxP system is used to perform site-directed recombination in 293 cells to produce Ad5 / F35-APE1 siRNA recombinant adenovirus with human APE1 gene siRNA expression sequence. The recombinant virus obtained in this way is a replication-deficient adenovirus with E1 deletion, and the virus can only realize the expression of foreign genes in cells that cannot provide the E1 region, but does not have the ability to proliferate. The packaging process of...
Embodiment 3
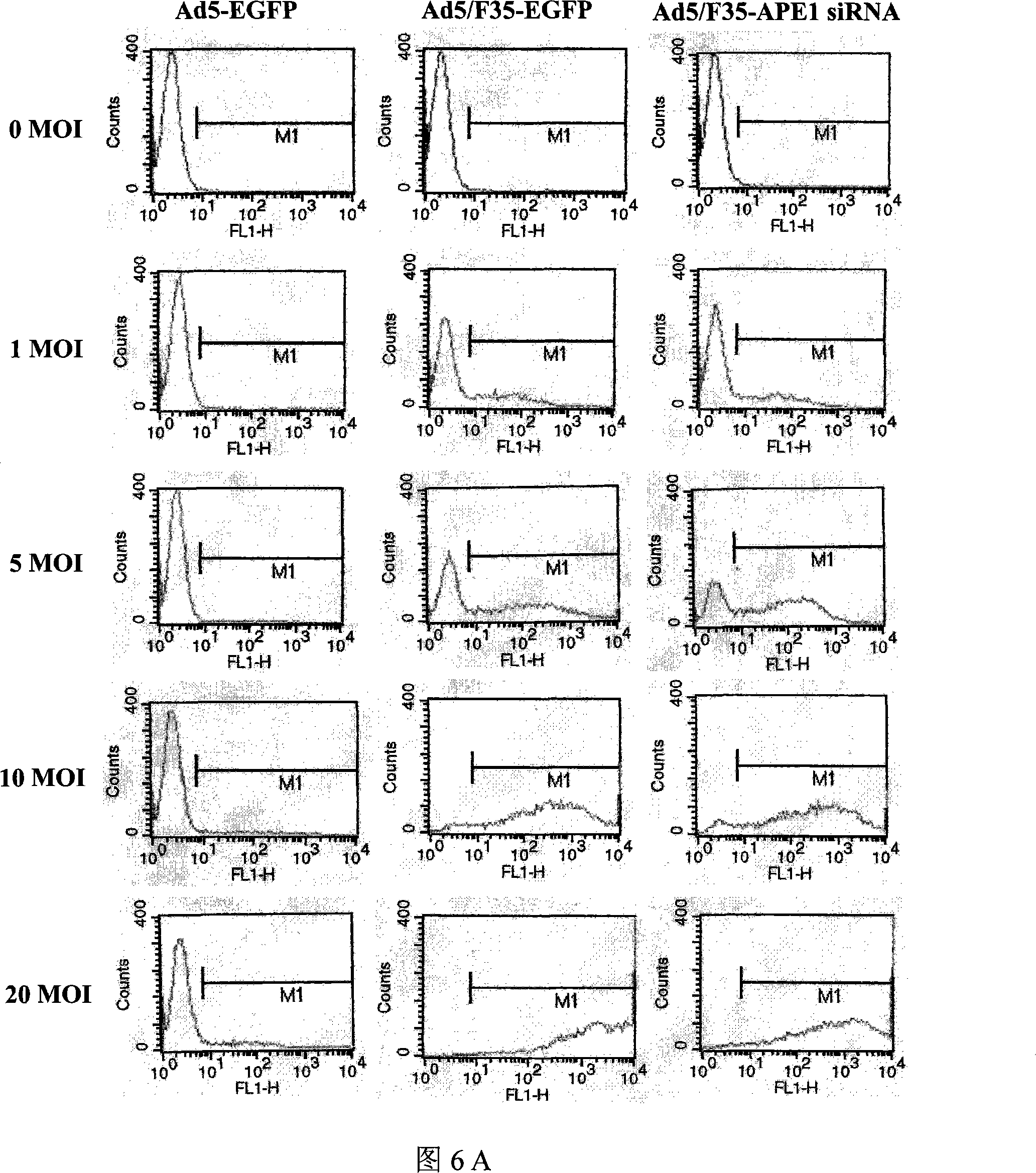
[0072] Example 3 Infection Efficiency of Ad5 / F35-APE1siRNA Recombinant Adenovirus to Colorectal Cancer Cells
[0073] 3.1 Detection of Infection Efficiency in Vitro
[0074] LOVO cells were cultured in an incubator containing 5% CO2 at 37°C, and the culture medium RPMI1640 contained 10% FCS, 1.0×10 5 U / L penicillin and streptomycin. The day before adenovirus infection, LOVO cells were seeded in a six-well plate, 5×10 per well 5 cells. Infect LOVO cells with adenovirus Ad5 / F35-APE1siRNA or control adenovirus Ad5 / F35-EGFP and Ad5-EGFP at a multiplicity of infection (MOI) of 0-20 for 90 min, then discard the culture medium and replace with complete medium After 24h, the expression of EGFP in LOVO cells was observed under a fluorescent inverted microscope and photographed. Digest the cells with 0.25% trypsin, centrifuge at 500g for 5min at room temperature, discard the supernatant, resuspend the cells in 0.01MPBS, centrifuge at 500g for 5min at room temperature, collect the ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com