Method for producing trichloro-acetic chloride with by-product in organic acyloxy silicone hydride production process
A technology of organic acyloxysilane and trichloroacetyl chloride, applied in the field of preparing trichloroacetyl chloride, can solve problems such as the outlet of by-product acetyl chloride, and achieve the effects of high conversion rate of raw materials, simple and easy production process, and few impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
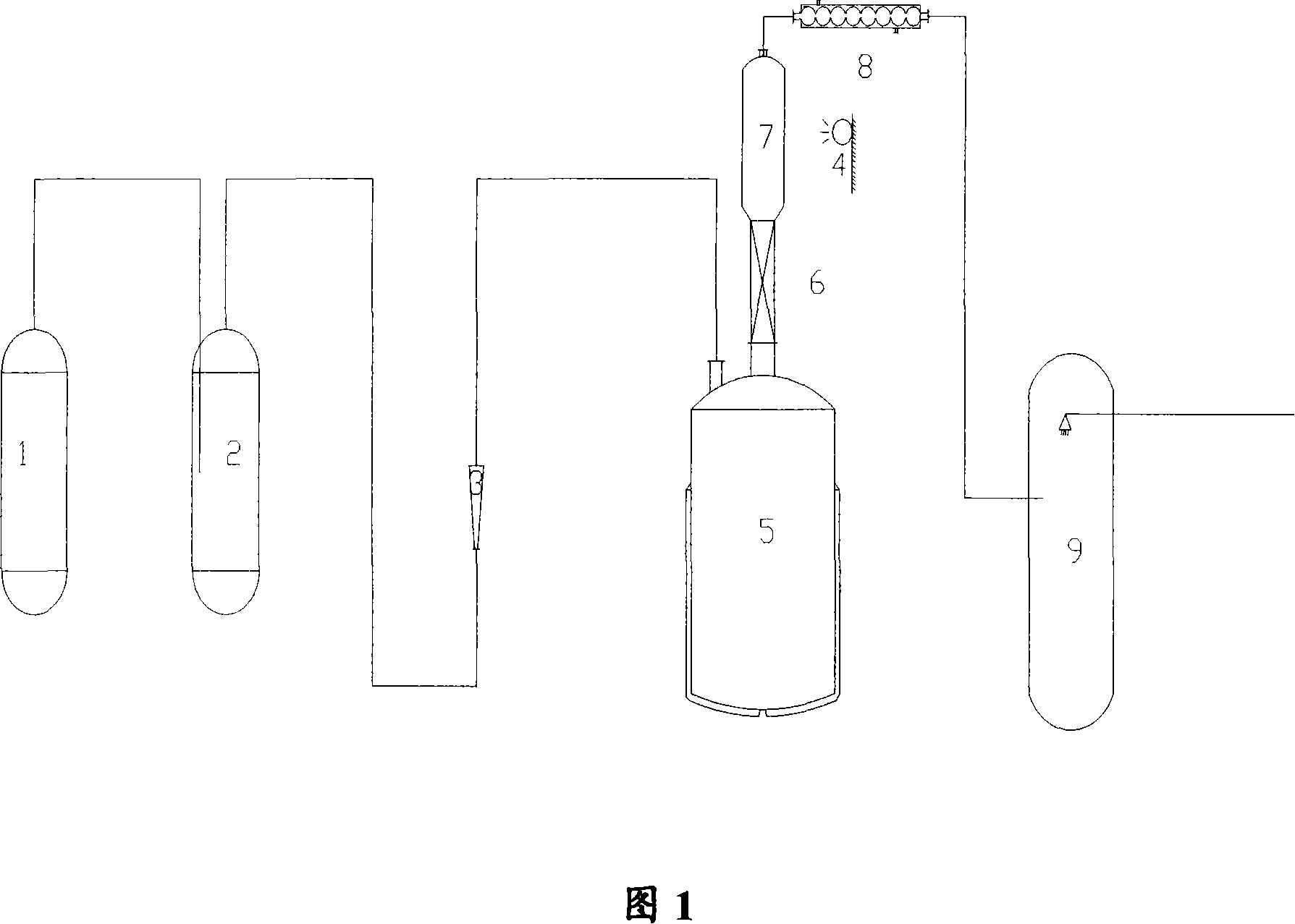
[0035]The reaction device is shown in Figure 1, and the method of the present invention adopts an ultraviolet lamp as the reactor light source. Add 700g of by-products in the production process of organoacyloxysilane to a 1000ml reactor, first pass a small amount of chlorine gas to replace the air in the reaction system, then control the temperature of the reactor at 70°C, and open the condensing reflux device. After the reaction kettle temperature and reflux in the system are stable, slowly open the chlorine gas valve, and the reaction proceeds immediately at this time. The temperature of the photochlorination gas phase reactor is 65° C. and there are liquid droplets on the wall of the reactor, and the tail gas is strongly acidic. At this time, the heating speed should be controlled to make the reflux moderate. At the same time, the flow rate of chlorine gas should be controlled so that the mass ratio of trichloroacetyl chloride to chlorine gas is about 1:4. As the reaction c...
Embodiment 2
[0038] The reaction device is shown in Figure 1, and an ultraviolet lamp is used as the reactor light source. Add 800g of by-products in the production process of organoacyloxysilane to a 1000ml reactor, first pass a small amount of chlorine gas to replace the air in the reaction system, then control the temperature of the reactor at 72°C, and open the condensing reflux device. After the reaction kettle temperature and reflux in the system are stable, slowly open the chlorine gas valve, and the reaction proceeds immediately at this time, the temperature of the photochlorination gas phase reactor is 67° C. and there are liquid droplets on the wall of the reactor, and the tail gas is strongly acidic. At this time, the heating speed should be controlled to make the reflux moderate, and the flow rate of chlorine gas should be controlled at the same time, so that the mass ratio of trichloroacetyl chloride to chlorine gas is about 1:6. As the reaction continues intensely, the temper...
Embodiment 3
[0041] The reaction device is shown in Figure 1, and an incandescent lamp is used as the reaction light source. Add 700g of by-products in the production process of organoacyloxysilane to a 1000ml reactor, first pass a small amount of chlorine gas to replace the air in the reaction system, then control the temperature of the reactor at 70°C, and open the condensing reflux device. After the reactor temperature and reflux in the system are stable, slowly open the chlorine gas valve, and the reaction proceeds immediately at this time, the temperature of the gas phase reactor is 62° C. and there are liquid droplets on the wall of the reactor, and the tail gas is strongly acidic. At this time, the heating rate should be controlled to make the reflux moderate, and the flow rate of chlorine gas should be controlled at the same time, so that the mass ratio of trichloroacetyl chloride to chlorine gas is about 1:6. As the reaction continues intensely, the temperature in the gas phase re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com