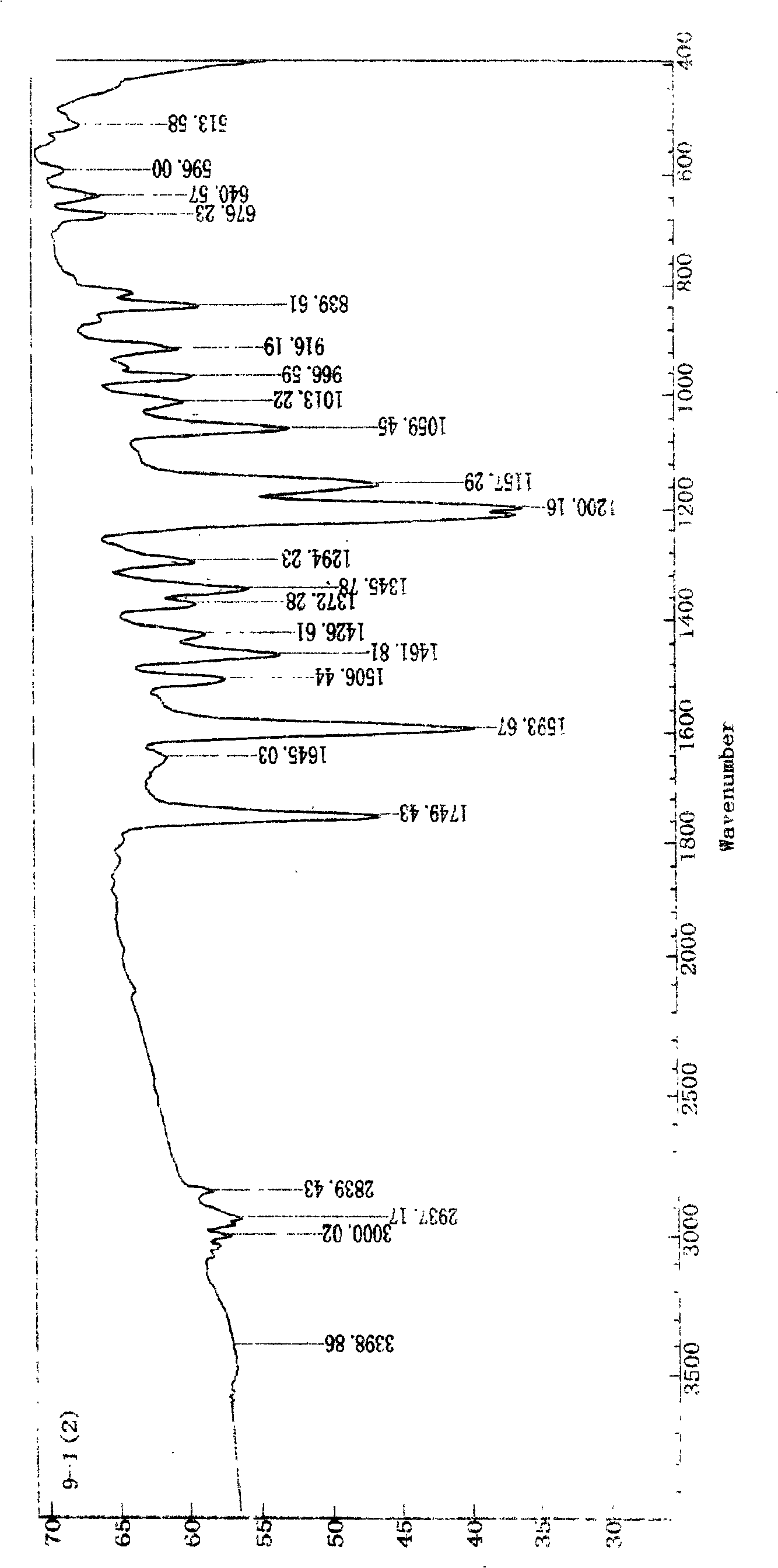
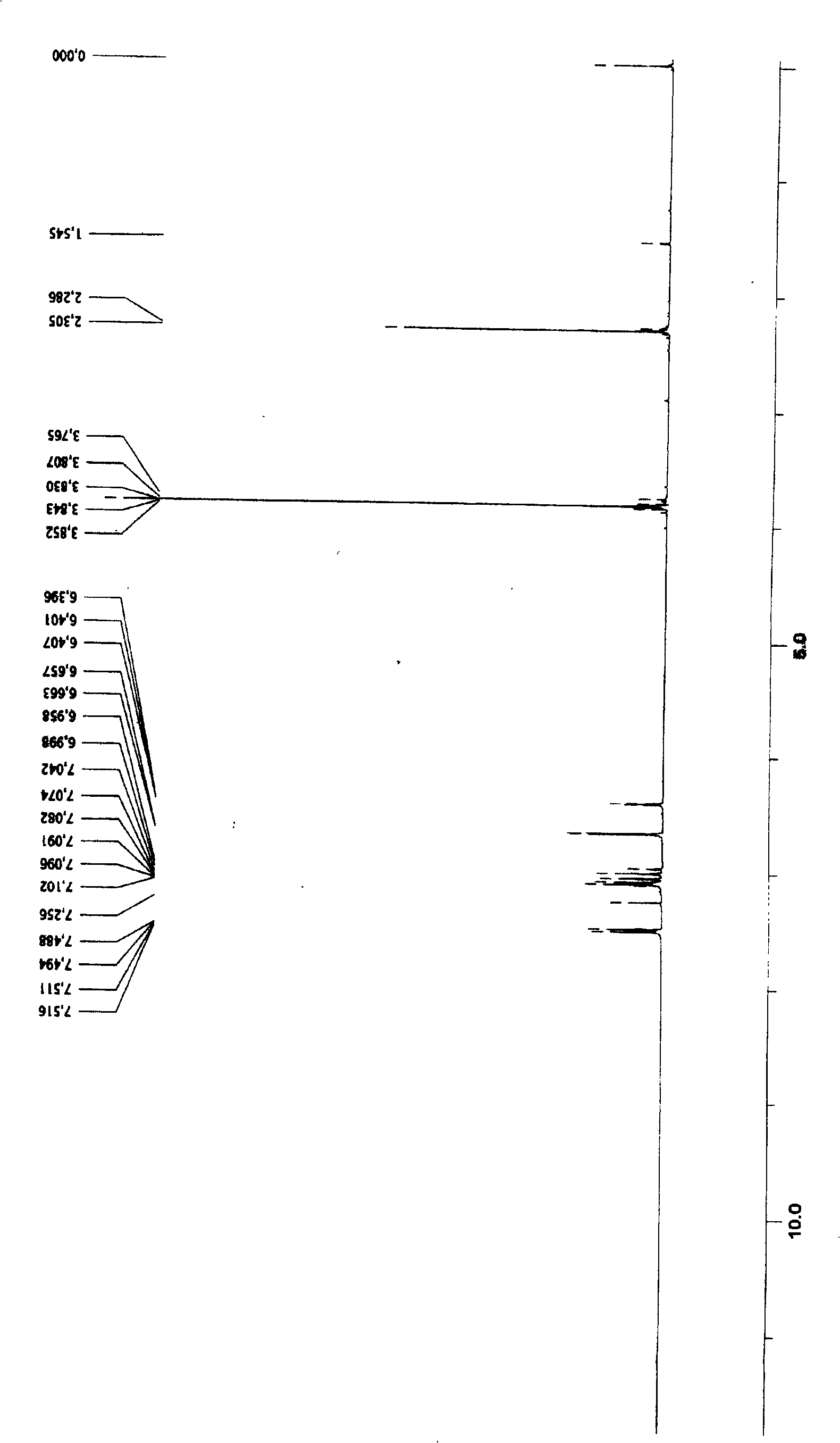
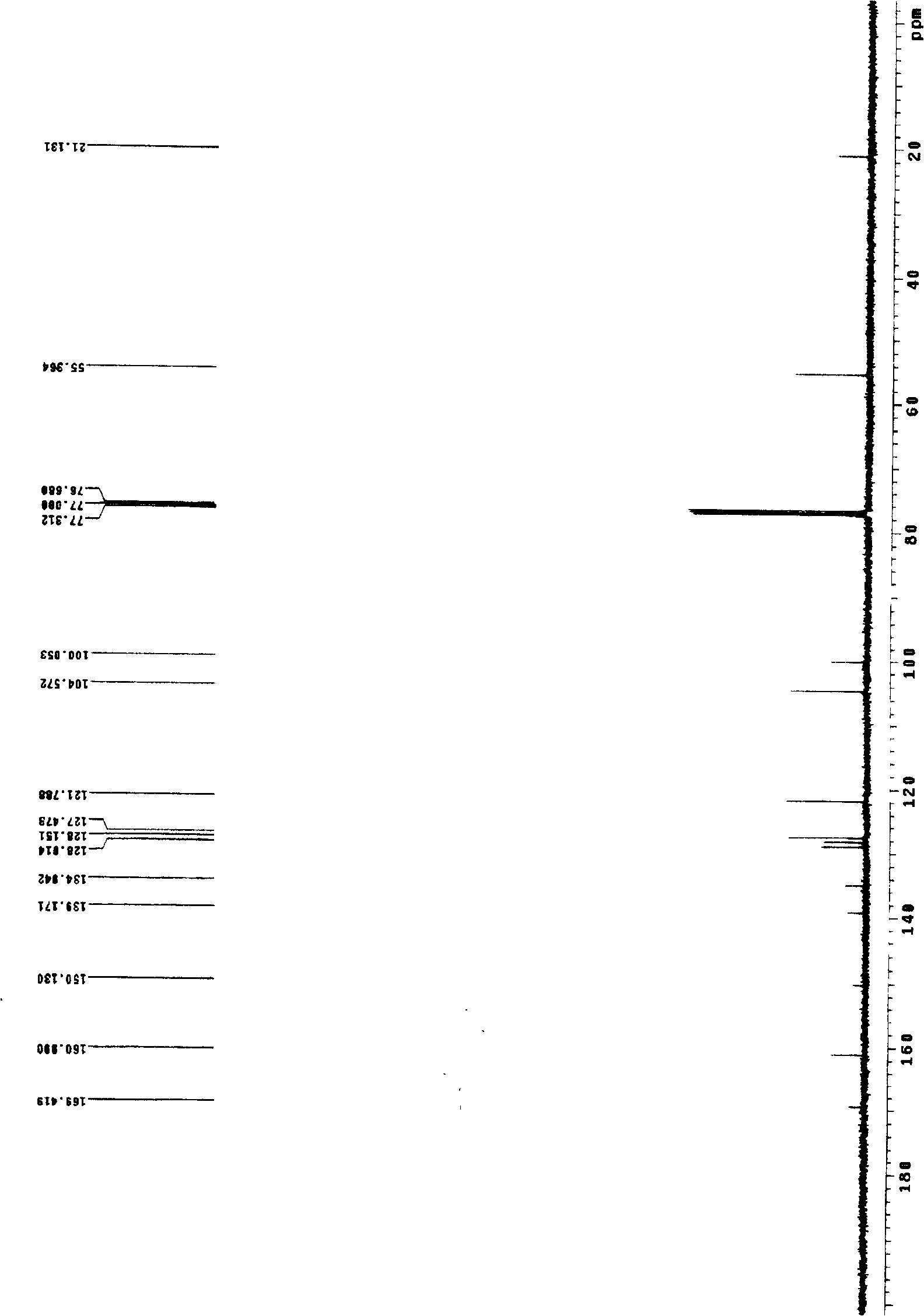
Process for producing (e)-3-dimethoxy-4'-acetoxy diphenyl ethylene
A technology of acetoxy stilbene and dimethoxy, which is applied in the field of preparation of (E)-3,5-dimethoxy-4'-acetoxy stilbene, can solve complex processes and production problems. The problems such as long cycle and impact on the ecological environment can achieve the effect of simple reaction steps, high total yield and environmental friendliness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] step one
[0029] Add 13.9g of p-cresol and 13.0g of acetic anhydride in a 100ml round-bottomed flask, stir vigorously with a magnetic stirrer, use a dropper to drop a drop of concentrated sulfuric acid as a catalyst, the reaction occurs immediately, and exotherm, continue to stir vigorously for 2 hours , stop the reaction, separate and purify the product by distillation under reduced pressure, distill out acetic acid at 45°C, collect the fraction at 114°C to obtain 17.5g of 4-acetoxytoluene (vacuum degree: 0.02MP) as a colorless transparent liquid, and the yield is 98%. .
[0030] Add 17.5g of 4-acetoxytoluene to another 250ml round-bottomed flask to react with NBS equivalent in quantity, use 50ml of carbon tetrachloride as a solvent, add 0.2g of benzoyl peroxide as an initiator, and heat at 80°C Heat and reflux for 5 hours, filter at 60-70°C, wash the insoluble matter with 10ml carbon tetrachloride three times, combine the organic phases, then evaporate the solvent c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com