Gel-typed polymer electrolyte containing diacryl amide-based polymeric material and electrochemical device comprising the same
A technology of diacrylamide and gel polymer, which is applied in the manufacture of electrolyte batteries, hybrid capacitor electrolytes, non-aqueous electrolyte batteries, etc. It can solve the problems of battery thickness expansion, electrolyte leakage, and difficulty in impregnation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Preparation of non-aqueous electrolyte containing lithium salt
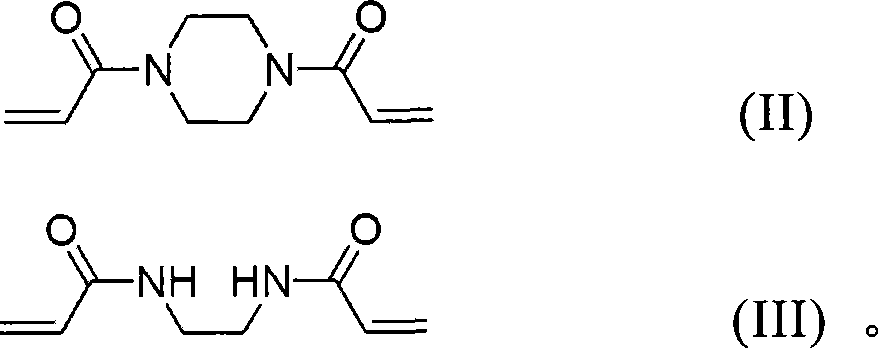
[0078] 1M LiPF 6 Added to a non-aqueous electrolyte solvent consisting of a mixture of ethylene carbonate (EC), ethylmethyl carbonate (EMC) and diethyl carbonate (DEC) (4:3:3, w / w). Based on the weight of the electrolyte, 1.5% by weight of vinylene carbonate (VC), 0.5% by weight of propylene sulfone (PS), 2 mol% of AIBN as a polymerization initiator, and 2% by weight of ethylenedipropylene represented by the aforementioned formula 3 An amide was added to the resulting mixture, thereby preparing a nonaqueous electrolyte.
[0079] Manufacture of cathode
[0080] Using LiCoO 2 As the cathode active material, 95.4% by weight of LiCoO with a particle size of 18 μm 2 , 1.6% by weight of Super-P (conductive material) and 3% by weight of PVDF (binder) as a cathode mixed material was added to NMP (N-methyl-2-pyrrolidone) as a solvent to prepare cathode slurry. Thereafter, the obtained cathode slurry was coa...
Embodiment 2
[0086] In addition to adding piperazine diacrylamide and polyethylene glycol diacrylate (Mn=700, Aldrich) in an amount of 2% by weight instead of ethylene diacrylamide, a secondary Battery.
experiment example 1
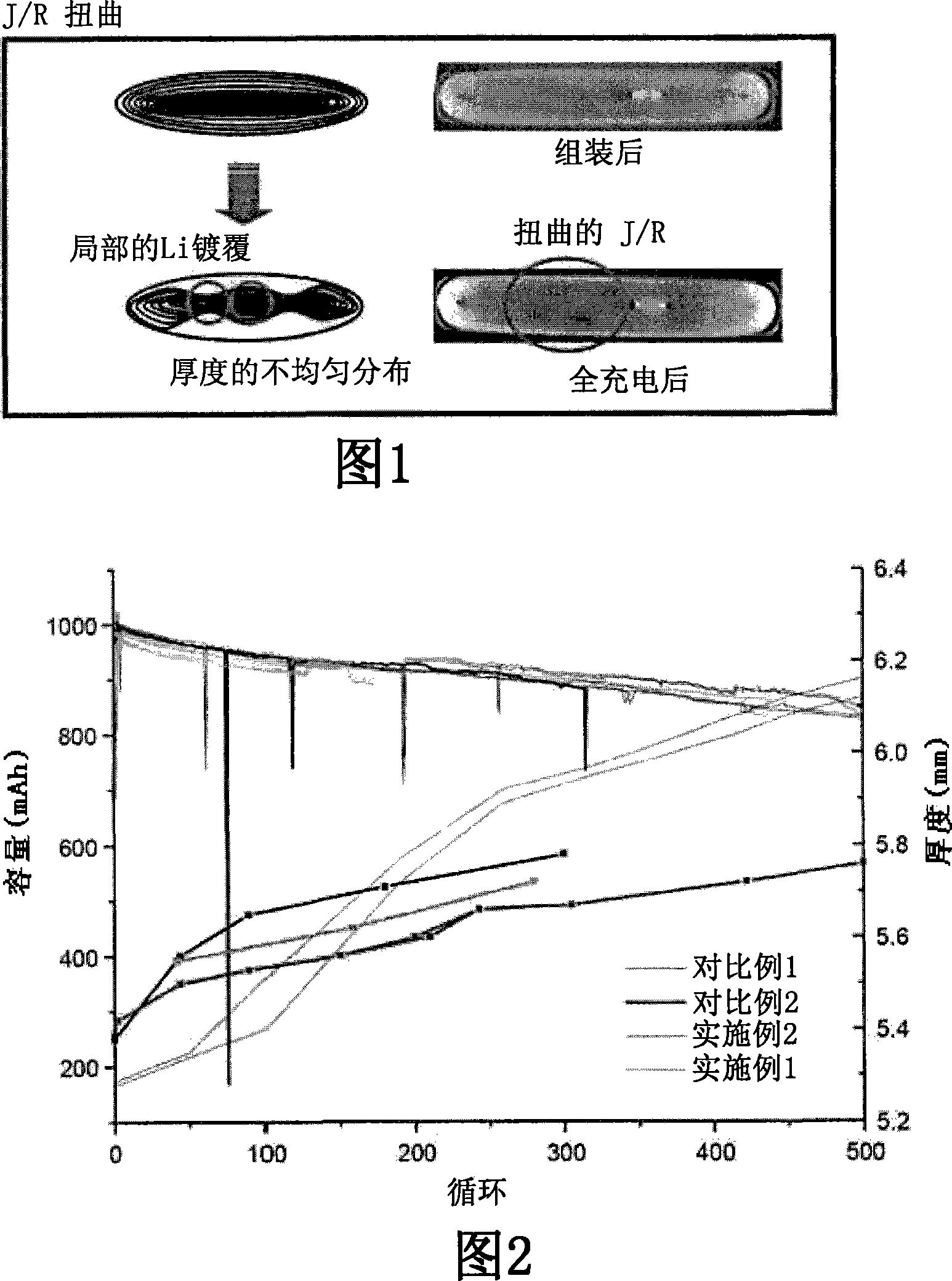
[0092] The batteries fabricated in Examples 1 and 2 and Comparative Examples 1 and 2 were charged to 4.2V at a charge rate of 1C, 50mA (off), and discharged to 3V (off) at a discharge rate of 1C. These charge / discharge cycles were repeated 500 times at room temperature. Meanwhile, in order to check the capacity of the battery, the manufactured battery was aged at room temperature for 5 days after its manufacture. Just after the battery capacity test, a cycle test is performed.
[0093] The changes in charge capacity and battery thickness of 300 to 500 cycles were measured respectively. The results thus obtained are shown in FIG. 2 .
[0094] As shown in Fig. 2, it can be seen that the batteries according to the present invention (Examples 1 and 2) exhibit almost comparable performance to that of the lithium-ion secondary battery (Comparative Example 2) even though it is a gel polymer battery system. The capacity of the lithium ion secondary battery of Comparative Example 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com