Method for preparing boron-carbon-zirconium material by liquid phase process
A liquid phase method, boron carbon technology, applied in the field of structural ceramics, can solve the problems of large particle size of boron carbon zirconium powder, uneven phase distribution of complex ceramics, difficult sintering, etc., and achieve the effect of overcoming relatively expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
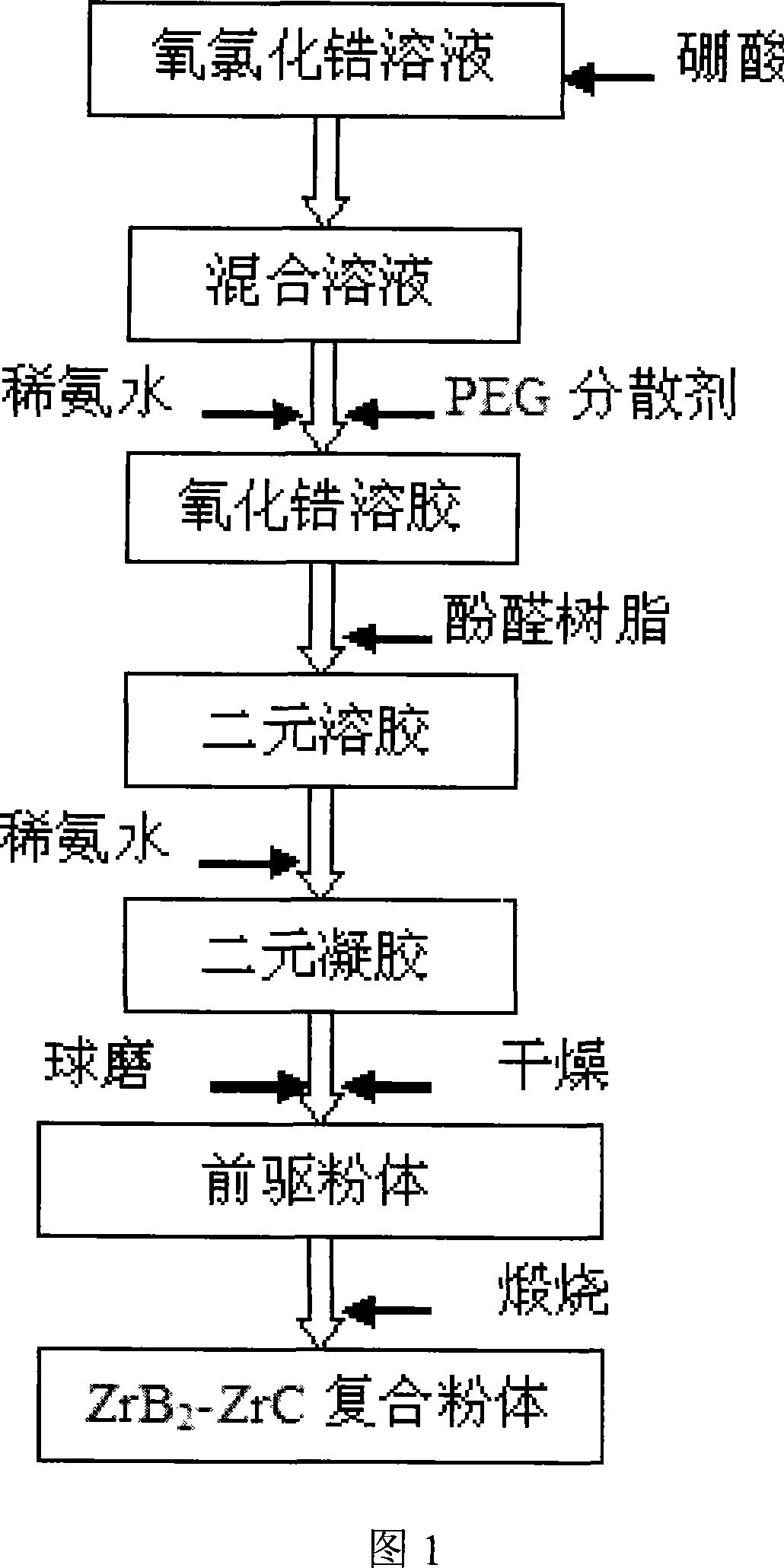
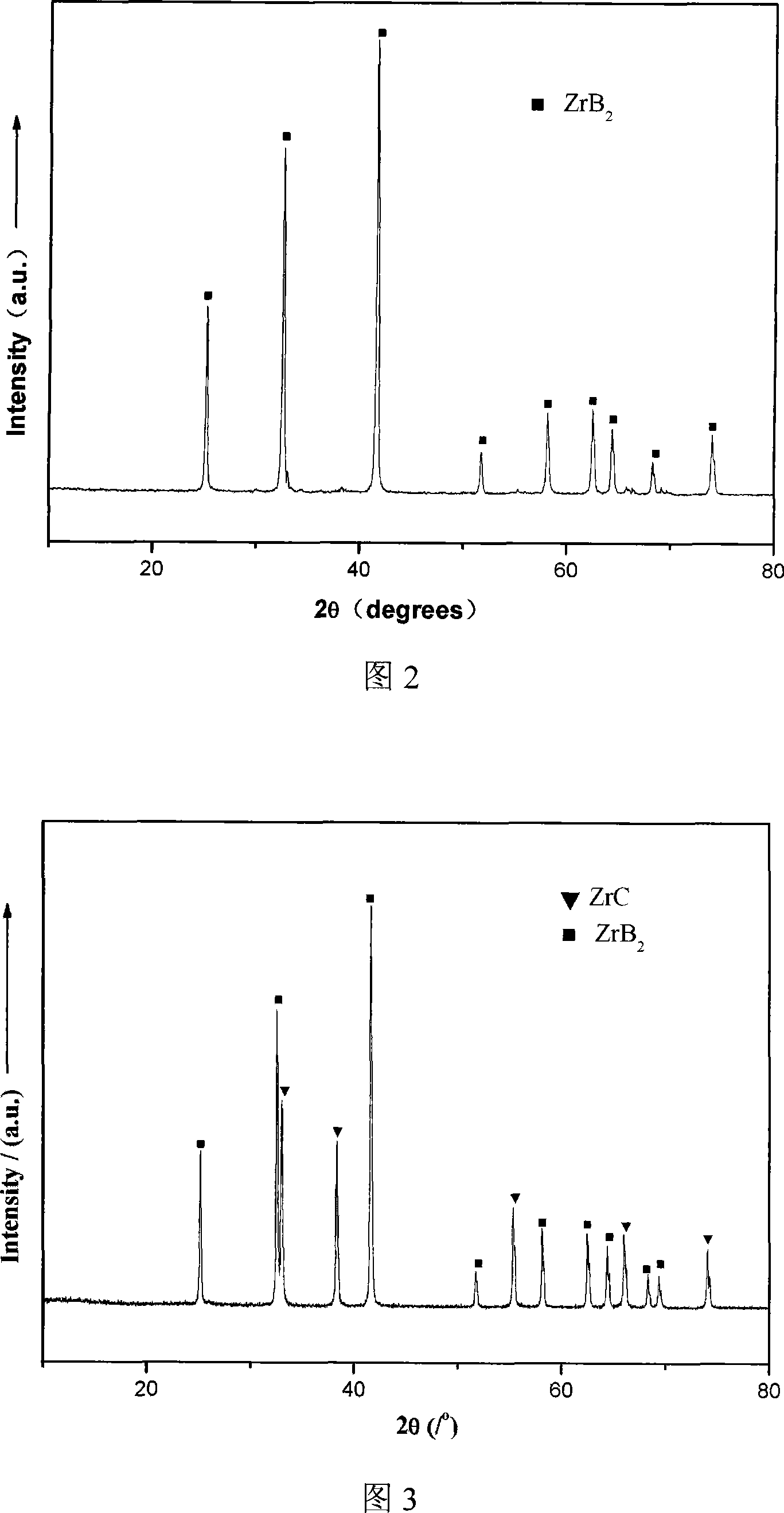
[0019] Dissolve 32.25g of zirconium oxychloride in 500ml of ethanol and water with a volume ratio of 4:1, and stir to dissolve it. Add 12.36g of boric acid and mix evenly, and add 3.60g of polyethylene glycol as a dispersant, then add 25vol% dilute ammonia water dropwise, adjust the pH to about 3, and form a zirconia sol. Add 20g of phenolic resin (the amount of residual carbon is about 50%, the concentration is 50vol%) to form a mixed sol. Continue to add ammonia water dropwise to make it condense. The gel was transferred to a ball mill jar, milled with zirconia as a ball mill for 24 hours, and dried at 80° C. for 12 hours to obtain a powder precursor. Calcined at 1500°C for 1 hour to obtain ZrB 2 Powder. It is obvious from the XRD pattern that ZrB 2 phase, no other intermediate phase exists. (See Figure 2) From the SEM photos, it can be seen that the powder is dispersed evenly, and the average particle size is about 50-200nm. (See Figure 5)
Embodiment 2
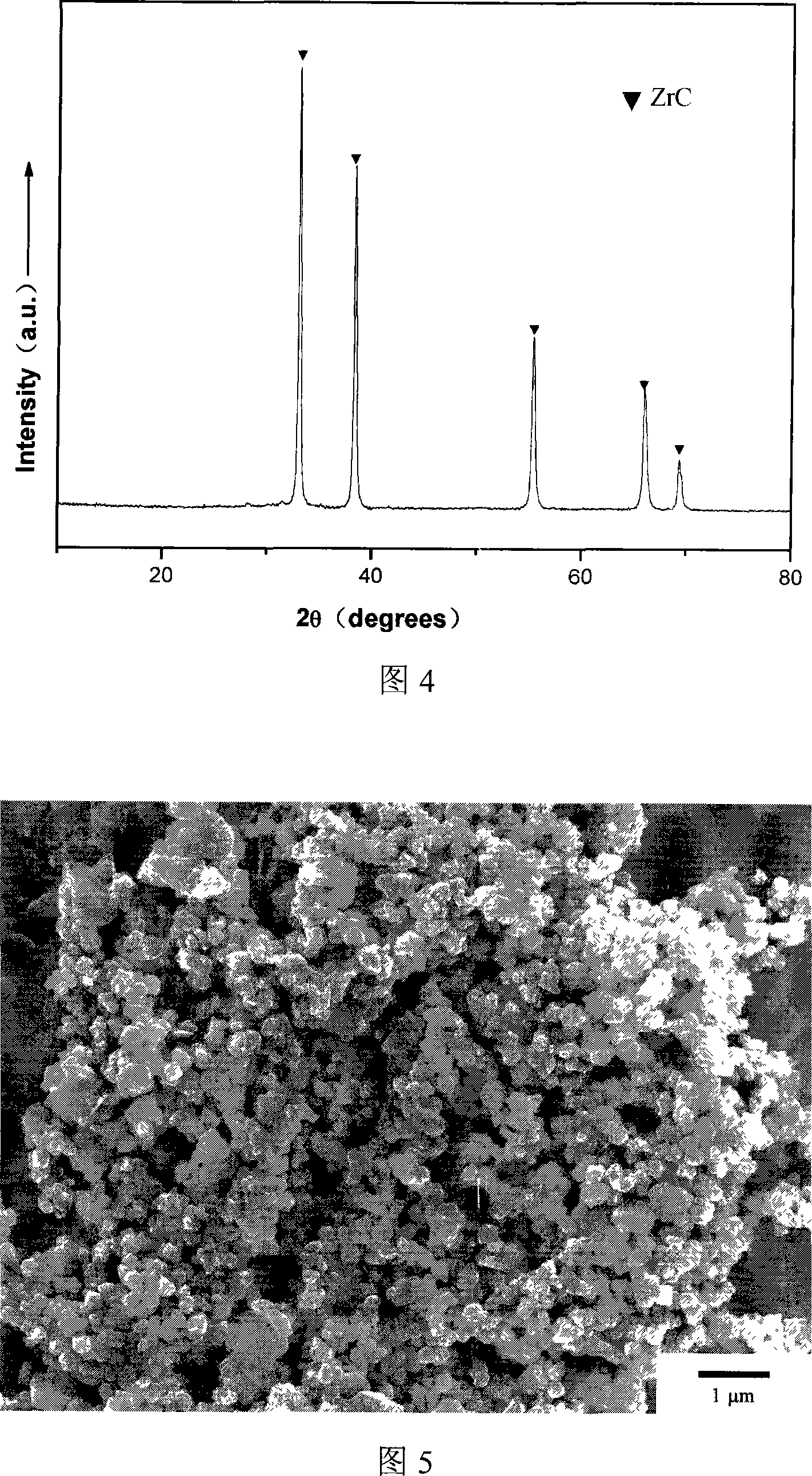
[0021] Dissolve 41.16g of zirconium oxychloride in 500ml of ethanol and water with a volume ratio of 4:1, and stir to dissolve it. Add 12.36g of boric acid and mix evenly, and add 4.80g of polyethylene glycol as a dispersant, then add 25vol% dilute ammonia water dropwise, adjust the pH to about 3, and form a zirconia sol. Add 23.28g of phenolic resin (the amount of residual carbon is about 50%, the concentration is 50vol%) to form a mixed sol. Continue to add ammonia water dropwise to make it condense. The gel was transferred to a ball mill jar, milled with zirconia as a ball mill for 24 hours, and dried at 80° C. for 12 hours to obtain a powder precursor. Calcined at 1500°C for 1 hour to obtain ZrB 2 -ZrC composite powder, the theoretical content of ZrC in the composite powder is 20wt%, and ZrB is clearly seen in the XRD figure 2 And ZrC phase exists, there is no other intermediate phase. (See Figure 3)
Embodiment 3
[0023] Dissolve 49.99g of zirconium oxychloride in 500ml of ethanol and water with a volume ratio of 4:1, and stir to dissolve it. Add 12.36g of boric acid and mix evenly, and add 4.90g of polyethylene glycol as a dispersant, then add 25vol% dilute ammonia water dropwise, adjust the pH to about 3, and form a zirconia sol. Add 26.55g of phenolic resin (the amount of residual carbon is about 50%, the concentration is 50vol%) to form a mixed sol. Continue to add ammonia water dropwise to make it condense. The gel was transferred to a ball mill jar, milled with zirconia as a ball mill for 24 hours, and dried at 80° C. for 12 hours to obtain a powder precursor. Calcined at 1500°C for 1 hour to obtain ZrB 2 -ZrC composite powder, the theoretical content of ZrC in the composite powder is 50wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com