Phenanthrene series derivatives and organic light-emitting diode containing the same
A derivative and organic technology, applied in the field of organic light-emitting diodes, can solve the problem that the energy gap is not enough to be used as organic phosphorescent molecules, and achieve the effects of improving carrier transport speed, best electroluminescent efficiency, and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Synthesis of compound 1:
[0068] 9,10: 9,10-bis(trimethylene)-9,10-dihydrophenanthrene (9,10:9,10-bis(trimethylene)-9,10-dihydrophenanthrene)
[0069] First, reactant 1 is prepared, and the reaction formula is as follows:
[0070]
[0071] Mix 2 grams of NaOH with 200 ml of methanol and heat to 60°C. After the NaOH is completely dissolved, add 3 grams of phenanthrene-9,10-dione and 4 grams of 1,3-acetonedicarboxylate (diethyl1,3-acetonedicarboxylate) (manufactured by ACROS, 95%), and maintain at 60°C . After 36 hours of reaction, 10% HCl aqueous solution was added to neutralize the precipitate and filter. The collected precipitate was dissolved in acetic acid, and then 300 ml of 10% HCl aqueous solution was added, and the reaction was heated for 18 hours. The acetic acid and water were removed, neutralized with sodium bicarbonate aqueous solution, and separated by precipitation and filtration to obtain the product of reactant 1 with a yield of 17%.
[0072] Next, compoun...
Embodiment 2
[0077] Synthesis of compound 2:
[0078] 9,10: 9,10-bis(trimethylene)-2,7-dibromo-9,10-dihydrophenanthrene (9,10:9,10-bis(trimethylene)-2,7-dibromo-9 , 10-dihydrophenanthrene)
[0079] The synthesis reaction formula is as follows:
[0080]
[0081] Dissolve 2 grams of compound 1 in 20 ml of dichloromethane (DCM) solvent, then add 20 ml of acetic acid solvent, drop 3 grams of bromine water (Br 2 (Manufactured by Lancaster), stirred at room temperature for three hours to obtain compound 2 as a white crystalline solid product, with a yield of 75%.
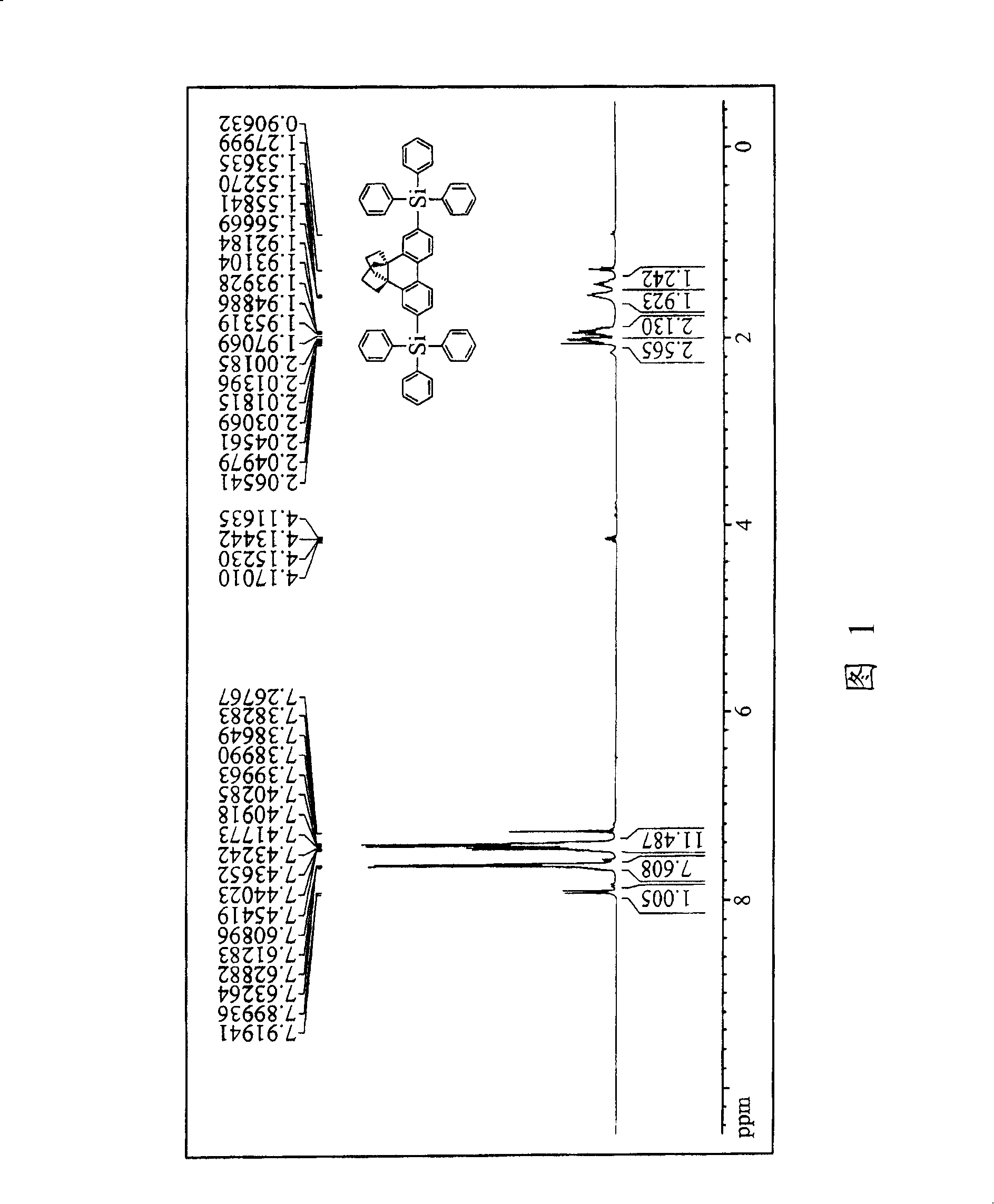
[0082] Measured for compound 2 1 H NMR(CDCl 3 ) The spectral data are as follows: δ(ppm) 1.44~1.48(m,2H), 1.57~1.65(m,2H), 1.92~1.99(m,4H), 2.10~2.16(m,4H), 7.31~7.33(d , 2H), 7.48 (s, 2H), 7.68-7.70 (d, 2H).
Embodiment 3
[0084] Synthesis of compound 3:
[0085] 9,10: 9,10-bis(trimethylene)-2,7-biscarbazole-9,10-dihydrophenanthrene (9,10:9,10-bis(trimethylene)2,7-biscarbazole-9 , 10-dihydrophenanthrene)
[0086] The synthesis reaction formula is as follows:
[0087]
[0088] Under nitrogen, 0.4 g of carbazole (manufactured by Aldrich, 96%) and 0.5 g of compound 2 were dissolved in 40 ml of o-xylene solvent, and 0.005 g of palladium (II) acetate (Palladium(II) acetate) was added. (Manufactured by Aldrich, 98%) catalyst, 0.32 g of sodium t-Butoxide (manufactured by Aldrich, 97%) and tri-tert-butyl phosphine (manufactured by Across, 99%) for reflux reaction In 12 hours, compound 3 was obtained as a white solid with a yield of 90%. This compound 3 is a novel phenanthrene derivative of the present invention.
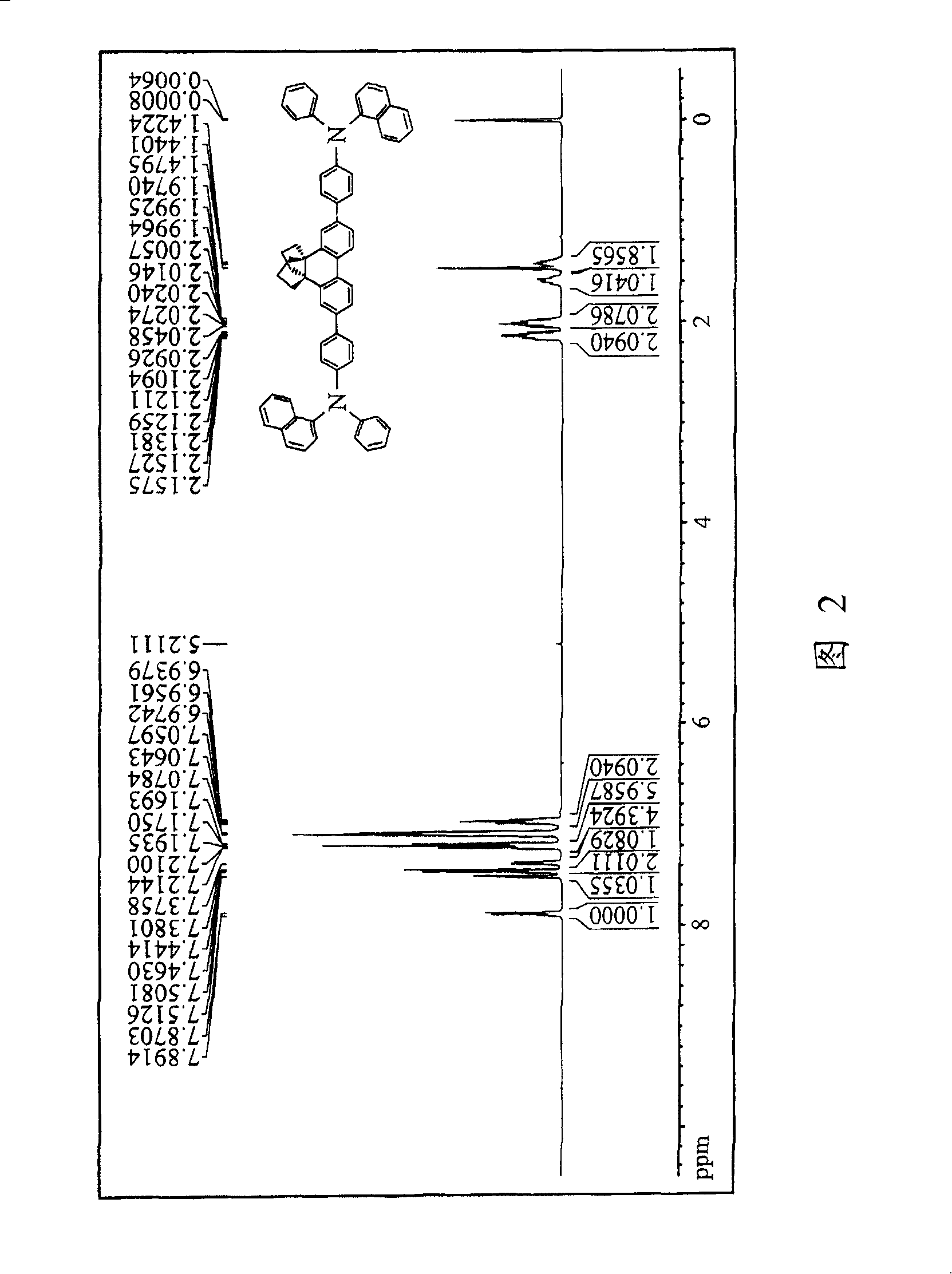
[0089] Compound 3 measured 1H NMR(400MHz, CDCl 3 ) The spectral data are as follows: δ (ppm) 1.58-1.64 (m, 2H), 1.69-1.75 (m, 2H), 2.15-2.23 (m, 4H), 2.22-2.29 (m, 4H), 7.30-7.33 (t , 4H), 7.44-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com