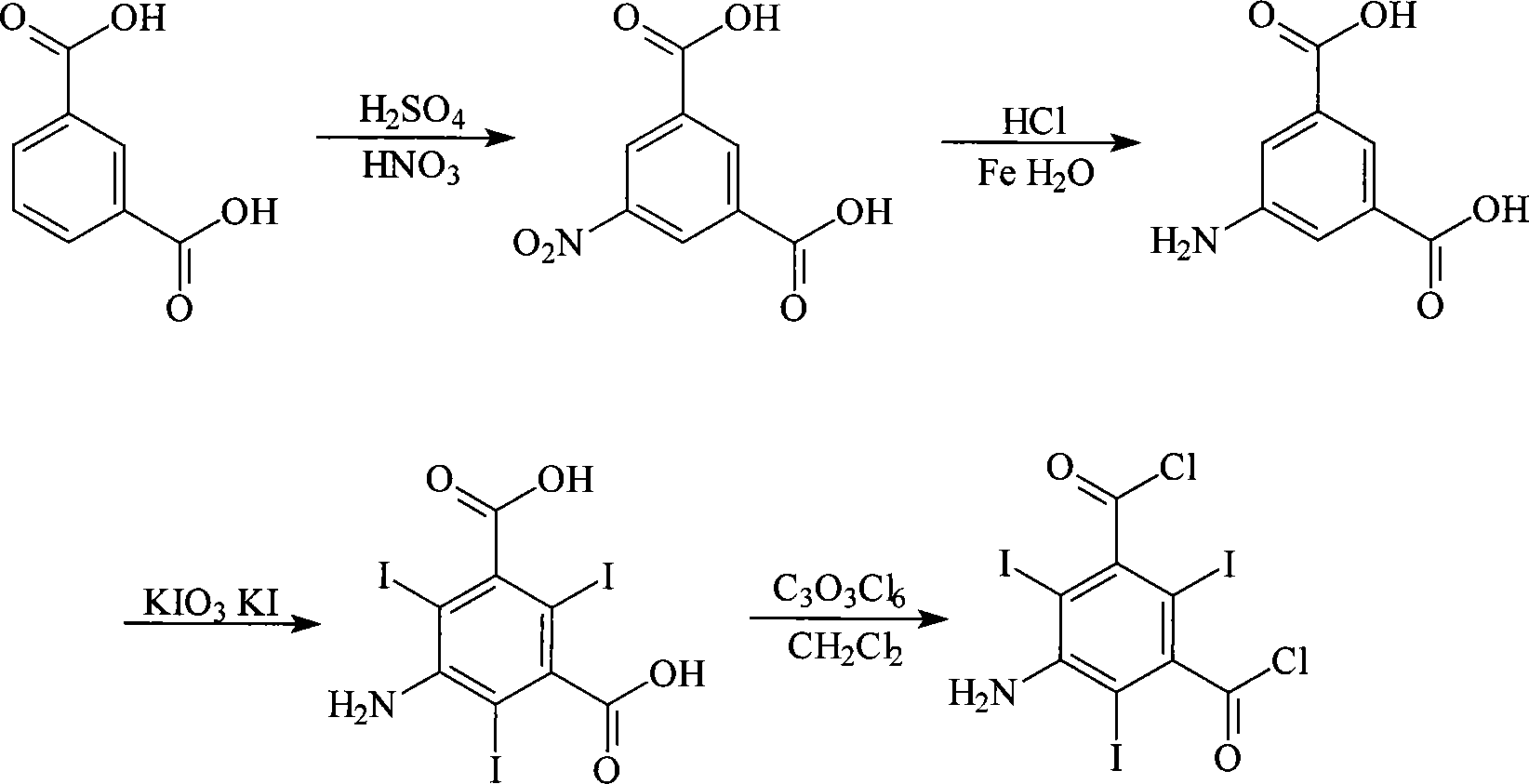
Method for synthesizing 5-amino-2,4,6-triiodoisophthaloyl dichloride
A technology for the synthesis of isophthalic acid, which is applied in the field of synthesizing non-ionic contrast agents, can solve problems such as unfavorable safety production, and achieve the effect of simplified operation and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of 5-nitroisophthalic acid
[0031] Add 100Kg of isophthalic acid into a dry 500L glass-lined reaction kettle, add 300kg of 98% concentrated sulfuric acid at room temperature, start stirring, slowly raise the temperature to 60°C, add 100kg of 95% concentrated nitric acid dropwise, and the dropping time is about 1h. During the dropwise addition, keep the temperature at 60-65°C. After the dropwise addition, the solution was slowly warmed up to 95° C., reacted at this temperature for 2 h, cooled to room temperature, and stirred for 12 h. Continue to cool the reaction material to 0-5°C, slowly add it into the water analysis kettle pre-installed with 0-5°C water and keep it warm for 1 hour, filter, wash and dry the filter cake, and obtain 112Kg of 5-nitroisophthalic acid as a white solid. The melting point is 265-267°C, the content is ≥99%, and the yield is 88%.
[0032] Preparation of 5-aminoisophthalic acid
[0033] Add 700Kg of water, 17Kg of 30% hydrochlor...
Embodiment 2
[0039] According to the method of Example 1, 5-aminoisophthalic acid was prepared.
[0040] 400Kg of water, 54.3Kg of 5-aminoisophthalic acid and 70L of 92% concentrated sulfuric acid were successively added into a 1000L glass-lined reactor. Add 96.5Kg of potassium iodate and 150Kg of potassium iodide to 350kg of 30% hydrochloric acid solution to prepare an iodo agent solution. At 30°C, the iodo agent solution was added dropwise into the reaction kettle within 3h, and then the temperature was raised to 80°C to keep the reaction for 10h. After the reaction, cool down to room temperature and filter to obtain 128.4Kg of 5-amino-2,4,6-triiodoisophthalic acid, melting point>300°C, content≥98.4%, yield 76.7%.
[0041] Add 350L of dichloromethane, 2Kg of triethylamine and 55.8Kg of 5-amino-2,4,6-triiodoisophthalic acid into a 500L dry glass-lined reactor in sequence, start stirring, and add 35.6kg of solid phosgene in batches at room temperature , During the feeding process, the te...
Embodiment 3
[0043] According to the method of Example 1, 5-amino-2,4,6-triiodoisophthalic acid was prepared.
[0044] Add 300L of dichloromethane, 10L of pyridine and 55.8Kg of 5-amino-2,4,6-triiodoisophthalic acid into a 500L dry glass-lined reactor in sequence, start stirring, and dissolve in 100L dichloroisophthalic acid at 30-35°C 89.1Kg of solid phosgene in methyl chloride was added to the reaction kettle within 5 hours, the temperature was raised to reflux, and the reaction was continued for 10 hours after the reaction solution became clear. Cool, add saturated Na dropwise to the reaction solution 2 CO 3 Solution, adjust to pH=7, let stand to separate layers, discard the water layer, add 100L water to wash the organic layer, discard the water layer, repeat the washing once, then concentrate the organic layer and recover dichloromethane, cool and filter , vacuum-dried the crystals to obtain 47.6 Kg of 5-amino-2,4,6-triiodoisophthalic acid chloride, the loss on drying was ≤0.5%, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com