Buflomedil production method
A technology of buflomedil and pyrrolidine, which is applied in the field of preparation of buflomedil, can solve problems such as high toxicity, and achieve the effects of short reaction time, high reaction yield and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
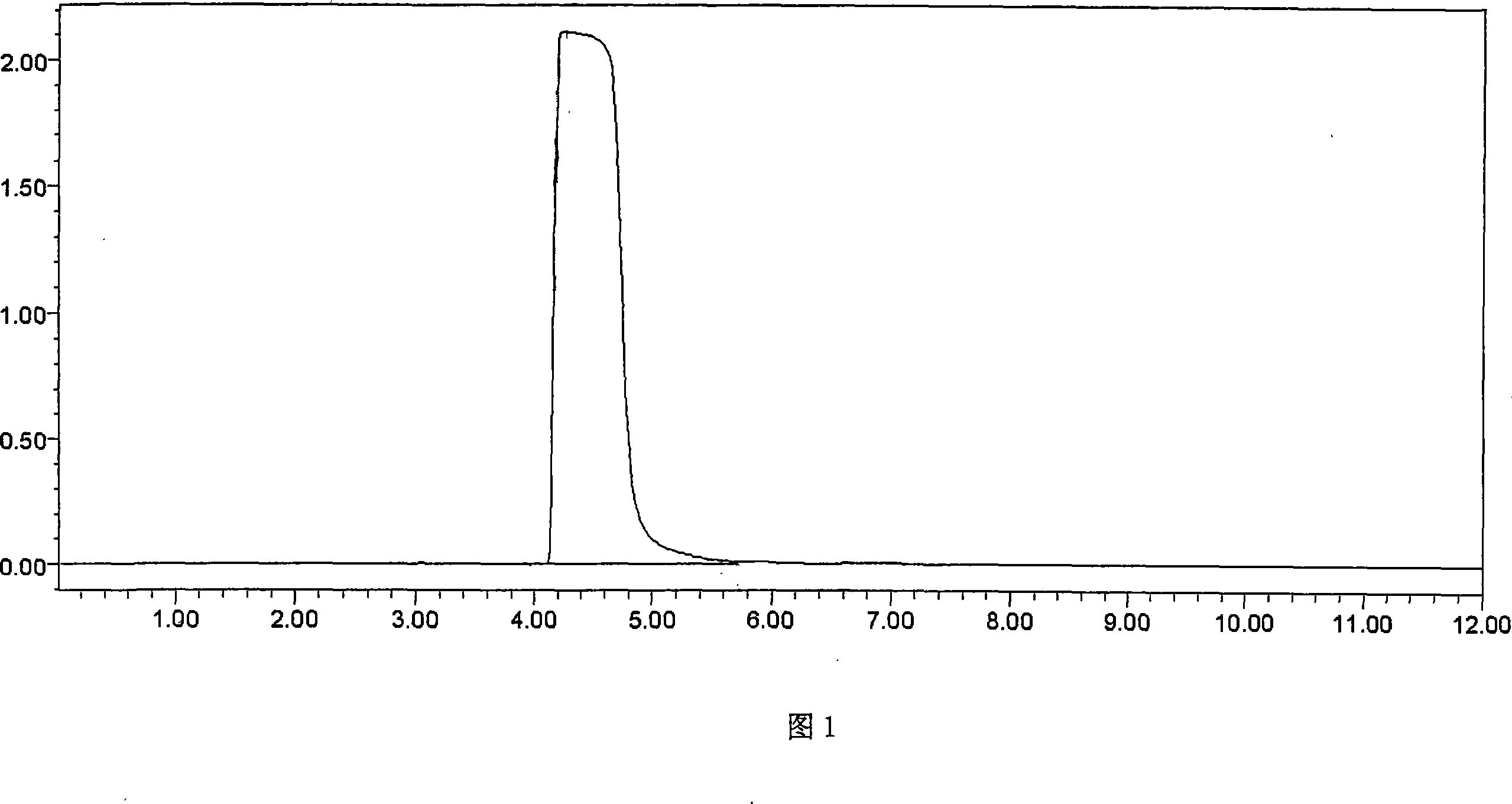
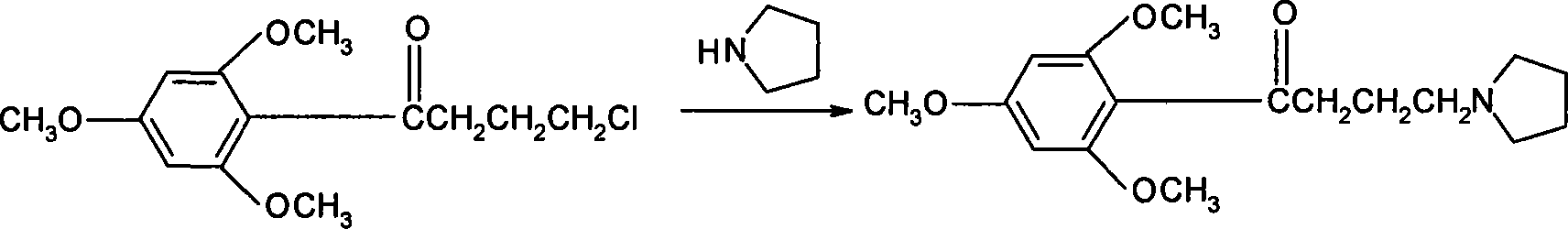
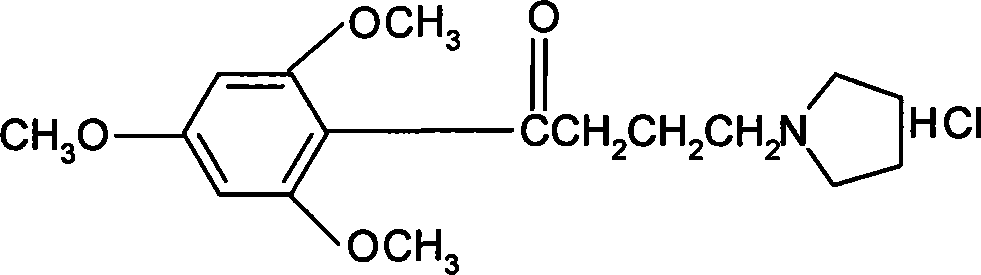
[0028] Put 50g of 4-chloro-1-(2,4,6-trimethoxyphenyl)butanone, 27g of pyrrolidine, 150g of cyclohexane, and 0.85g of sodium iodide into a 1000ml reaction flask successively, heat to reflux, and keep the temperature for reaction. After 5 hours, the temperature was lowered to 35°C, and the organic layer was washed three times with 150 ml of 20% sodium chloride solution, and then the organic layer was depressurized and concentrated to obtain 50.7 g of buflomedil with a content of 99.0% (HPLC). The yield was 90.24%.
Embodiment 2
[0030] Put 50g of 4-chloro-1-(2,4,6-trimethoxyphenyl)butanone, 30g of pyrrolidine, 250g of cyclohexane, and 0.85g of sodium iodide into a 1000ml reaction flask in sequence, heat to reflux, and keep the temperature for reaction. After 9 hours, the temperature was lowered to below 35°C, and the organic layer was washed three times with 150ml of 20% sodium chloride solution, and then the organic layer was depressurized and concentrated to obtain 52.52g of buflomedil with a content of 98.5% (HPLC). The yield was 93.5%.
Embodiment 3
[0032] Put 50g of 4-chloro-1-(2,4,6-trimethoxyphenyl)butanone, 18g of pyrrolidine, 300g of cyclohexane, and 0.85g of sodium iodide into a 1000ml reaction flask in sequence, heat to reflux, and keep the reaction temperature warm. After 12 hours, the temperature was lowered to below 35°C, and the organic layer was washed three times with 150 ml of 20% sodium chloride solution, and then the organic layer was depressurized and concentrated to obtain 50.7 g of buflomedil with a content of 98.5% (HPLC). The yield was 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com