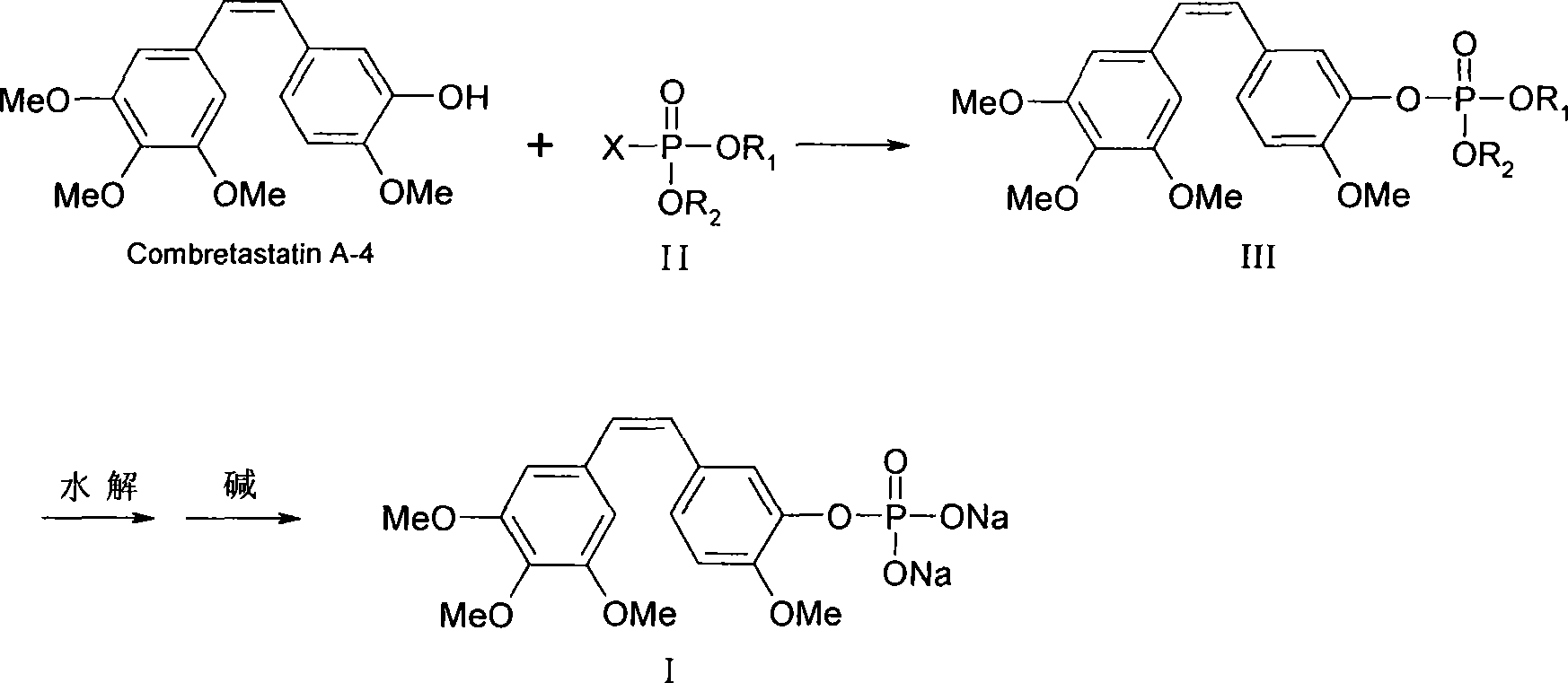
Method for preparing Combretastatin A-4 phosphoric acid ester disodium salt
A technology of compretin and phosphate ester, which is applied in the field of preparation of compretin A-4 phosphate disodium salt, can solve the problems of complicated purification methods, low yield, reduced yield and the like, and achieves easy industrial production. , The effect of high product yield and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
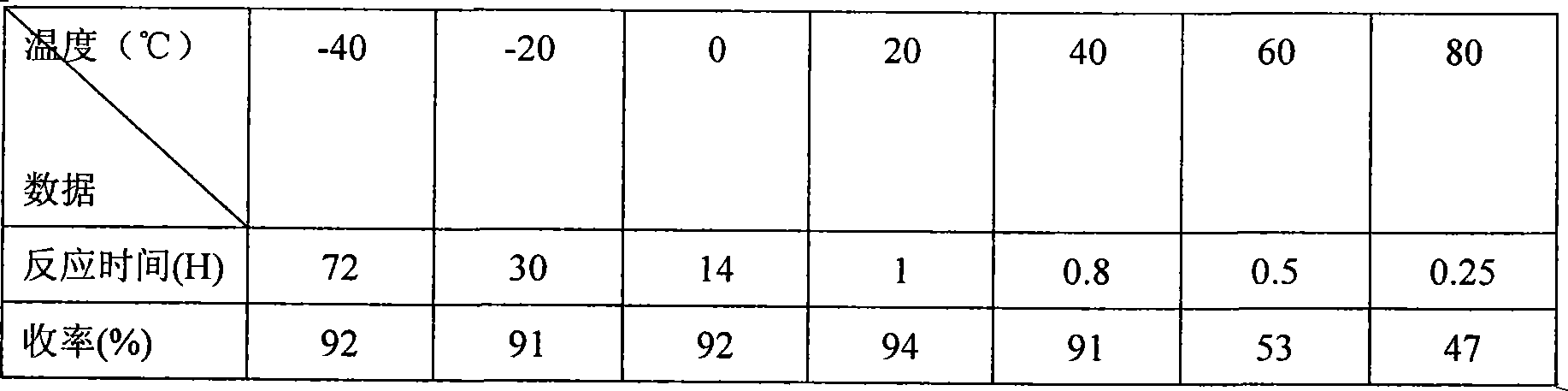
[0033] The investigation of embodiment 1 temperature and reaction time:
[0034] According to literature reports, the conventional CA4P synthesis reaction is carried out at low temperature, and the reaction takes a long time, which is not conducive to effective control of the reaction process. This experiment is a comprehensive verification of the reaction time and temperature, as follows:
[0035] Under nitrogen protection, 3.16g (0.01mol) of CA4, 0.06g (0.0005mol) of 4-N,N-lutidine and 1.42g (0.011mol) of diisopropylethylamine were dissolved in 20ml of dichloromethane, A solution of 2.51 g (0.011 mol) of di-(tert-butoxy)-phosphoryl chloride and 10 ml of dichloromethane was added dropwise at different temperatures. After the dropwise addition, keep the temperature and stir the reaction, monitor the reaction process with thin-layer chromatography, until the raw material point disappears, pour the reaction solution into 50ml ice water, separate the organic layer, extract the wa...
Embodiment 2
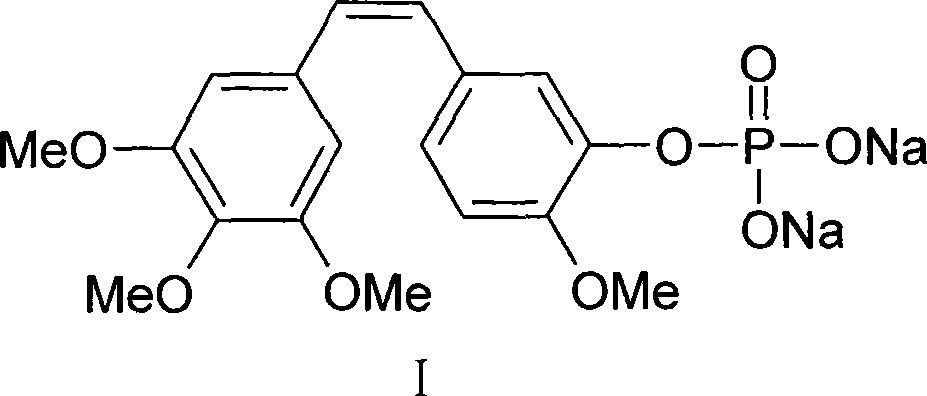
[0038]The preparation of embodiment 2 Compretastatin Combretastatin A-4 phosphate disodium salt
[0039] Under nitrogen protection, dissolve 3.16g (0.01mol) of CA4, 0.06g (0.0005mol) of 4-N,N-lutidine and 1.42g (0.011mol) of diisopropylethylamine in 20ml of dichloromethane , at 20°C, a solution of 2.51 g (0.011 mol) of di-(tert-butoxy)-phosphoryl chloride and 10 ml of dichloromethane was added dropwise. After the dropwise addition, keep the temperature and stir the reaction, monitor the reaction process with thin-layer chromatography, until the raw material point disappears, the reaction time is 0.5 hour, the reaction solution is poured into 50ml ice water, the organic layer is separated, and the water layer is refilled with 20ml diethyl alcohol. Methyl chloride was extracted once, the organic layers were combined, washed with water, dried, and evaporated to dryness to obtain a yield of 94% oil CA4-two-(tert-butoxy)-phosphate 4.77g, and put it into 50ml of pre-dried hydrogen c...
Embodiment 3
[0041] Embodiment 3 Preparation of Combretastatin Combretastatin A-4 Phosphate Disodium Salt
[0042] Under nitrogen protection, dissolve 3.16g (0.01mol) of CA4, 0.06g (0.0005mol) of 4-N,N-lutidine and 1.42g (0.011mol) of diisopropylethylamine in 20ml of dichloromethane , at 30° C., a solution of 2.45 g (0.011 mol) of bis-(2-cyanoethoxy)-phosphoryl chloride and 10 ml of dichloromethane was added dropwise. After the dropwise addition is completed, keep the temperature and stir the reaction, monitor the reaction process with thin layer chromatography, until the raw material point disappears, the reaction time is 0.8 hours, the reaction solution is poured into 50ml of ice water, the organic layer is separated, and the water layer is re-used with 20ml of two Extracted once with methyl chloride, combined the organic layers, washed with water, dried, and evaporated to dryness to obtain a yield of 93% oil CA4-two-(2-chloroethoxy)-phosphate 5.14g, which was dropped into 25ml of methan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com