Near-infrared absorbing material and use thereof
A technology of near-infrared rays and absorbing materials, which is applied to radiation-absorbing coatings, other chemical processes, instruments, etc., and can solve problems such as low solubility and degradation of resin compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0272] [Production Example 1] Synthesis of Compound 1
[0273] [chem 21]
[0274] Compound 1
[0275] Under nitrogen flow, in a four-necked flask, add phenylglyoxal monohydrate (glyoxal) (4.56g, 30mmol), bromoethylbenzene (11.1g, 60mmol), dichloroethane 60ml, and dropwise slowly therein Titanium tetrachloride (8.54 g, 45 mmol) was stirred at room temperature for 3 hours. After the reaction was completed, water was added for extraction, evaporated, and recrystallized from hexane to form the above-mentioned compound 1. The yield was 46%.
manufacture example 2
[0276] [Production Example 2] Synthesis of Compound 2
[0277] [chem 22]
[0278]
[0279] The benzoin derivative compound 1 (3.0 g, 9.4 mmol) synthesized in Production Example 1, phosphorus pentasulfide (12.53 g, 28.2 mmol), and 50 ml of dioxane were added to a 4-neck flask, and refluxed for 2 hours under a nitrogen atmosphere. After the reaction, filter and add NiCl to the filtrate 2 .6H 2 O (1.12 g, 4.7 mmol) aqueous solution was again refluxed for 2 hours. After the reaction was completed, water and methanol were added, stirred for a period of time, and then filtered to recover the above-mentioned compound 2. The yield is 45%.
manufacture example 3
[0280] [Production Example 3] Synthesis of P-1
[0281] [chem 23]
[0282]
[0283] Compound 2 (0.8 g, 1.06 mmol) synthesized in Production Example 2, bisphenol A (0.24 g, 1.06 g) and K were added to a 4-necked flask 2 CO 3 (1.16g, 8.44mmol), DMF40ml, stirred at 80°C for 8 hours under nitrogen atmosphere. After the reaction was completed, it was filtered and evaporated, then dropped into a water / methanol=1 / 2 solution, and a small amount of HCl aqueous solution was added for washing. After filtration, the target object P-1 was recovered. The yield is 50%.
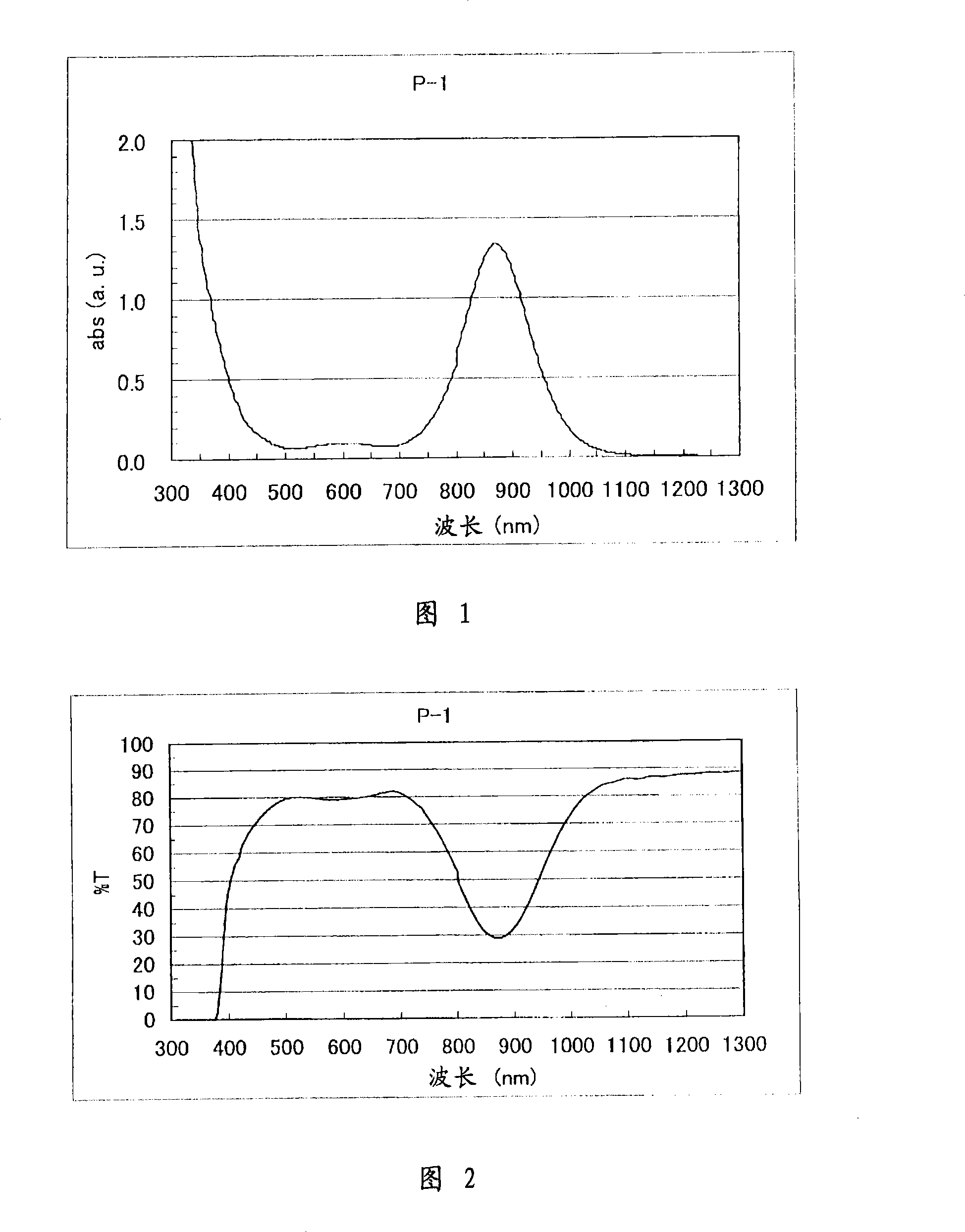
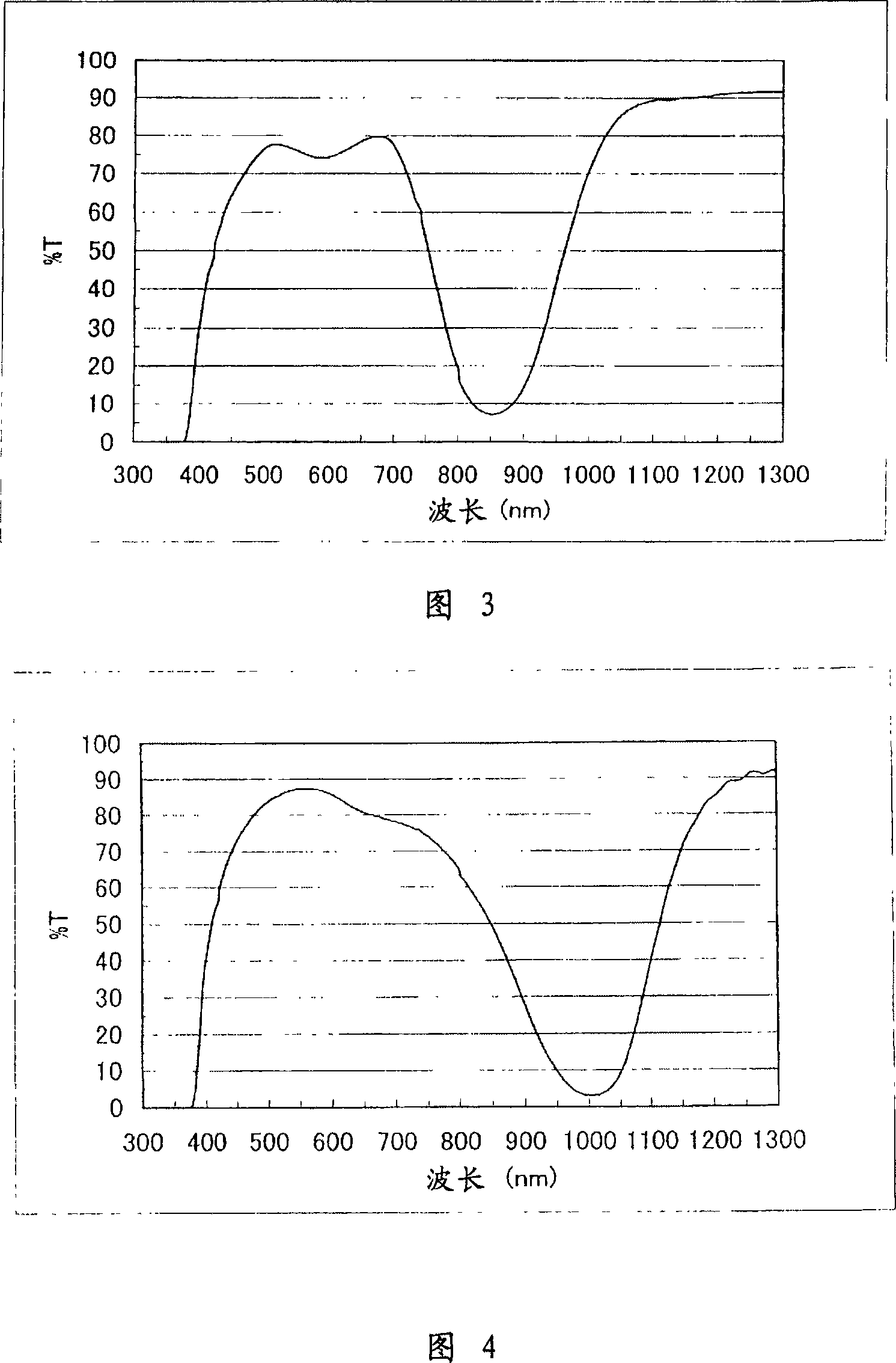
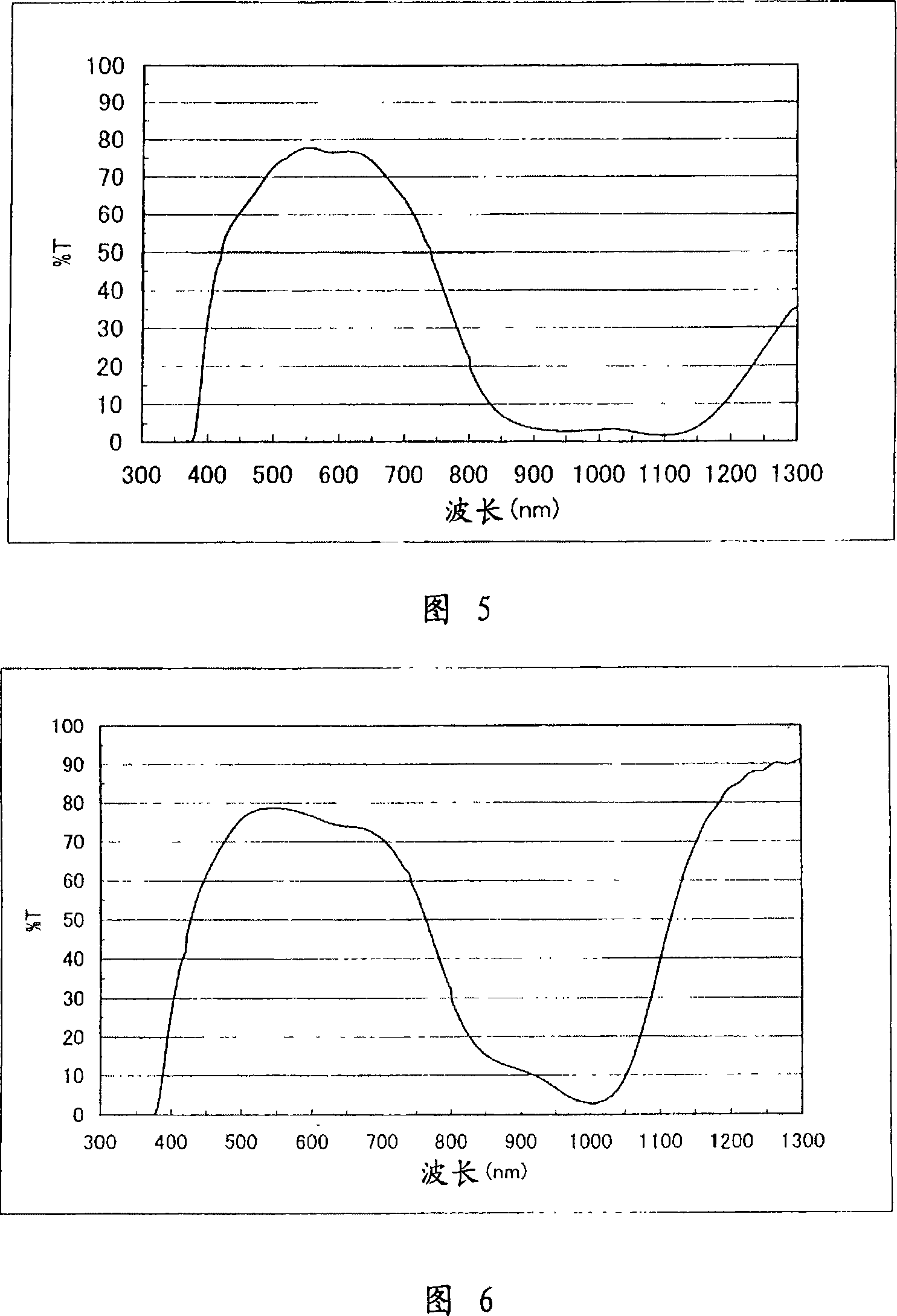
[0284] Figure 1 shows the absorption spectrum of P-1 in chloroform. It can be seen from Figure 1 that P-1 absorbs less visible light and has greater absorption in the near-infrared range.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com